Hydrothermal, sol-gel, electrodeposition, chemical precipitation, and biosynthesis are top methods used in the synthesis of manganese dioxide to produce high-purity material. The purity, crystallinity, and shape of manganese dioxide, achieved through these synthesis techniques, significantly influence its performance in batteries, catalysis, and nanomaterials. Demand for advanced manganese dioxide continues to grow globally each year:
Aspect | Details |
|---|---|
Market Size 2023 | |
Projected Market Size 2032 | About USD 2.8 billion |
CAGR (2023-2032) | 7.2% |
Primary Demand Drivers | Batteries (lithium-ion, alkaline), mostly for EVs and portable electronics |
Key End-User Industries | Cars (EV manufacturing), Electronics (capacitors, small parts), Chemical (oxidizing agent) |
Emerging Applications | Water purification, renewable energy storage |
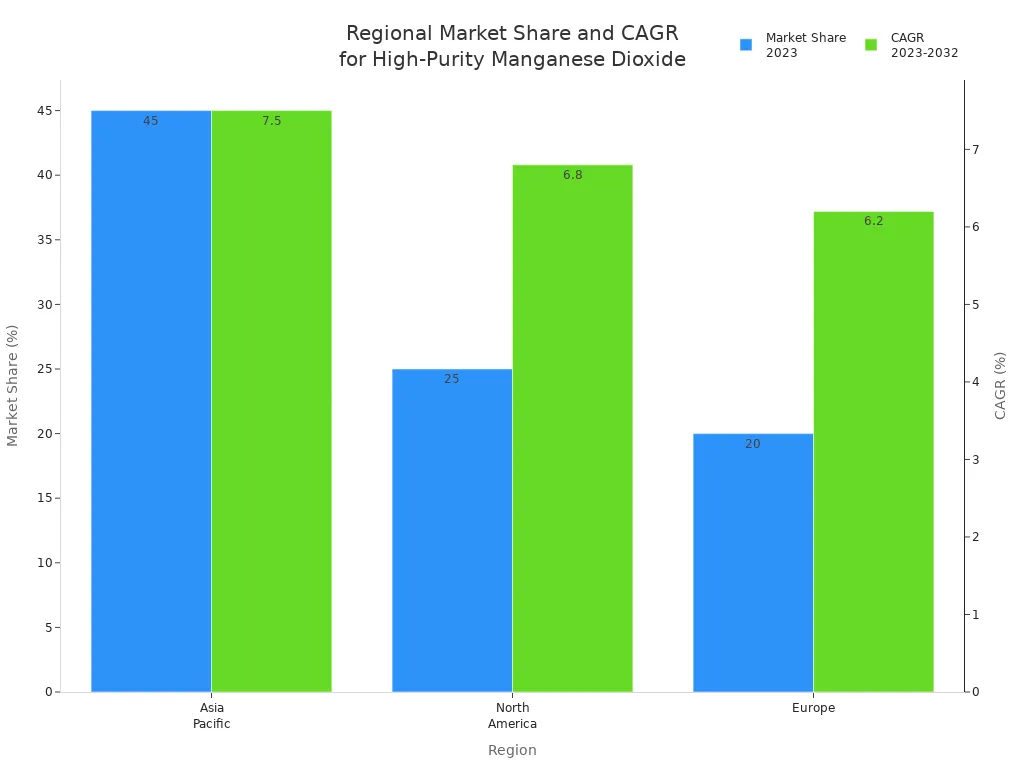
Regional Market Shares 2023 | Asia Pacific: 45%, North America: 25%, Europe: 20% |
Regional Growth Projections | Asia Pacific CAGR about 7.5%, North America about 6.8%, Europe about 6.2% |
Market Challenges | Fluctuating raw material prices, competition from alternative materials |
Growth Opportunities | Increasing EV adoption, expanding renewable energy storage, advancements in electronics |
Picking the best way to make manganese dioxide is important. Methods like hydrothermal or biosynthesis help get pure manganese dioxide. This pure manganese dioxide works well in batteries, imaging, and treatments.
Green and new ways, like biosynthesis and microwave-assisted synthesis, are good for the environment. They also save money and make manganese dioxide with special shapes. These shapes help it work better.
Changing the size, shape, and purity of manganese dioxide nanomaterials makes them work better. This helps in energy storage, catalysis, and medical uses.
Synthesis of Manganese Dioxide
Choosing the right way to make manganese dioxide is important. The method you pick changes how pure it is and what its crystals look like. These things affect how well manganese oxide nanomaterials work in batteries and imaging. Scientists use different ways to control these features for special uses.
Hydrothermal and Sol-Gel Methods
Hydrothermal and sol-gel methods are common for making manganese dioxide. They help get very pure and well-formed crystals. Hydrothermal synthesis uses heat and pressure in a closed container. This makes crystals that are thick and even. X-ray tests show hydrothermal synthesis gives α-MnO2 with no extra stuff mixed in. The crystal shape matches the hollandite-type tetragonal phase. If you wait longer, the crystals get squeezed but stay pure.
Sol-gel methods often use a template to shape the nanomaterials. This helps make even particles that are very pure. But taking out the template can add unwanted things. Clumping during heating is also a problem. Sol-gel takes more time than hydrothermal but lets you control the size better.
Synthesis Method | Purity Level | Crystallinity Characteristics | Additional Notes |
|---|---|---|---|
Hydrothermal | Good purity (no impurity peaks) | High crystallinity; uniform and controllable crystal growth; complete crystal form; α-MnO2 phase confirmed by XRD with no impurity peaks | Requires complex high temperature and pressure environment; produces dense crystal lattice under compressive stress with increased reaction time |
Sol-gel (often with template) | High purity | Uniform dispersion; high purity but potential agglomeration during calcination; long reaction period | Template removal can introduce impurities; agglomeration is a challenge during calcination process |
These methods help make manganese oxide nanomaterials with special features. Hydrothermal synthesis is good for the environment and saves money. Sol-gel lets you make nanosheets and other shapes.
Electrodeposition and Chemical Precipitation
Electrodeposition and chemical precipitation are easy ways to make manganese dioxide. These ways are simple and can be used for big batches. Electrodeposition uses electricity in a solution with manganese ions. Manganese dioxide forms on the electrode. Scientists can change the purity and shape by changing the current, time, and pH.
Parameter/Condition | Values/Range | Effect on MnO2 Purity and Performance |
|---|---|---|
10 ms | High specific capacitance and good electrochemical performance | |
Pulse current toff | 30 ms |
|
Frequency | 25 Hz |
|
Duty cycle (θ) | 25% |
|
Peak current density (Ip) | Not explicitly stated |
|
Pulse current ton (Li-MnO2) | 5 ms | Optimal for lithium manganese dioxide synthesis with high discharge capacity |
Pulse current toff (Li-MnO2) | 45 ms |
|
Duty cycle (θ) (Li-MnO2) | 10% |
|
Peak current density (Ip) | 1 mA dm⁻² |
|
pH of electrodeposition bath | 7 | MnO2/RGO nanocomposite with superior capacitance and stability |
Current density | 200 A m⁻² | Optimal morphology and electrochemical performance (5 h deposition) |
Deposition time | 5 h |
|
Using the right current and time helps get the best purity and shape. Chemical precipitation uses a reaction to make manganese dioxide from a solution. This way is cheap and good for making a lot. But if the starting materials are not pure, it can add bad stuff. Washing and filtering help remove these extras.
Thermal Decomposition and Roasting
Thermal decomposition and roasting are old ways to make manganese dioxide. These ways use heat to change manganese compounds into manganese dioxide. Manganese carbonate is often used first. Heating it between 334 °C and 412 °C breaks it into MnO and CO2. Mixing it with iron oxide and heating above 650 °C makes manganese ferrite. Roasting at 750 °C changes MnO2 and Fe2O3 into new forms.
Stage | Reaction / Transformation | Temperature Range (°C) | Precursor Materials / Notes |
|---|---|---|---|
1 | MnO2 → Mn2O3 | 460–570 | MnO2 polymorphs (α-, β-, γ-, δ-, λ-MnO2) |
2 | Mn2O3 → Mn3O4 | 700–800 | Thermal decomposition continues |
3 | Mn3O4 → MnO | >1200 | Final reduction product |
– | MnO2 polymorphs | N/A | β-MnO2 (pyrolusite) most abundant; α-MnO2 has open tunnel structures |
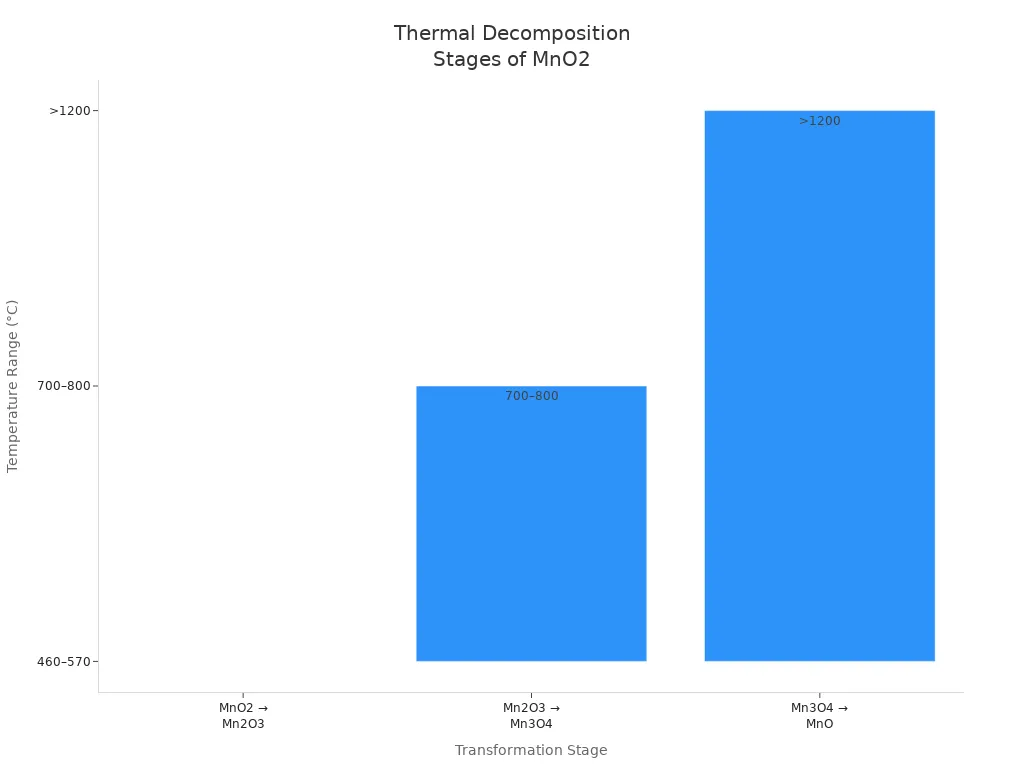
Thermal decomposition lets you make manganese dioxide with different crystal shapes. The starting material and heat level decide the final product. This way is good for the environment and big batches, but you might need to clean it more.
Template and Microwave-Assisted Methods
Template and microwave-assisted methods give more control over making manganese oxide nanomaterials. Templates help shape nanosheets, hollow balls, and other forms. Hard templates like polystyrene and soft ones like chiral molecules make special shapes. Some MnO2 templates can remove themselves and are easy to use. These shapes help with imaging and treatment by giving more surface area.
Microwave-assisted synthesis uses microwaves to heat things fast and evenly. This saves energy and makes the reaction quicker. It also helps get more manganese out of ores, which saves money. You must control the microwave settings carefully. This way is good for the environment and helps make nanomaterials with better features for imaging and treatment.
Aspect | Microwave-Assisted Method Advantages | Limitations / Notes |
|---|---|---|
Heating Efficiency | Direct penetration of microwave energy into materials, leading to rapid and uniform heating. | Requires specific microwave equipment and frequency control (commonly 2450 MHz). |
Energy Consumption | Lower energy consumption compared to conventional heating due to selective and rapid heating. | Initial setup cost and scale-up challenges may exist. |
Leaching Rate | Achieved Mn extraction rate of 93.03% in 3 hours, which is 17.19% higher than conventional heating (75.84%). | Optimal parameters (pyrite ratio, acid concentration, temperature) must be carefully controlled. |
Reaction Mechanism | Suppresses formation of sulfur passivation layer on MnO2 surface via dipole rotation and directional motion of molecules. | Limited direct information on template methods for MnO2 synthesis in the source. |
Environmental Impact | Reduces amount of reducing agent and sulfuric acid needed; shorter reaction times; environmentally friendly process. | Specific to leaching from pyrolusite, not general synthesis of high-purity MnO2. |
Application Scope | Successfully applied in hydrometallurgy for recovery of manganese, copper, nickel, and chalcopyrite ores. | No direct data on template-assisted synthesis methods for MnO2 purity enhancement. |
Hydrothermal synthesis without a template can also change the shape of nanomaterials. Changing the reaction time makes different shapes, like β-MnO2 microncubes. These shapes help imaging and treatment by absorbing more electromagnetic waves.
Manganese Oxide Nanomaterials
Making manganese oxide nanomaterials needs careful control of size and shape. Surfactants like Pluronic help make nanoparticles. The PEO to PPO ratio in the surfactant changes the size and how the particles group. Heating after making them, and how much oxygen is there, changes the crystal type and strength.
Changing the amount of Mn-MOF and KMnO4 and the pH changes the nanomaterial’s structure. More Mn-MOF makes smaller particles with bigger surfaces. Different pH levels make rods, needles, or flower-like nanosheets. These shapes help imaging and treatment by making the material work better.
MnO2 nanomaterials often do not conduct electricity well and have small surface areas. Pure MnO2 breaks down during redox cycling, so it does not last long. Mixing MnO2 with carbon nanomaterials like graphene helps it conduct better and last longer. Making special shapes, like porous balls or nano-cones, increases how much it can hold and how fast ions move.
Synthesis Method / Modification | Morphology / Structure | Electrochemical Impact | Key Findings / Metrics |
|---|---|---|---|
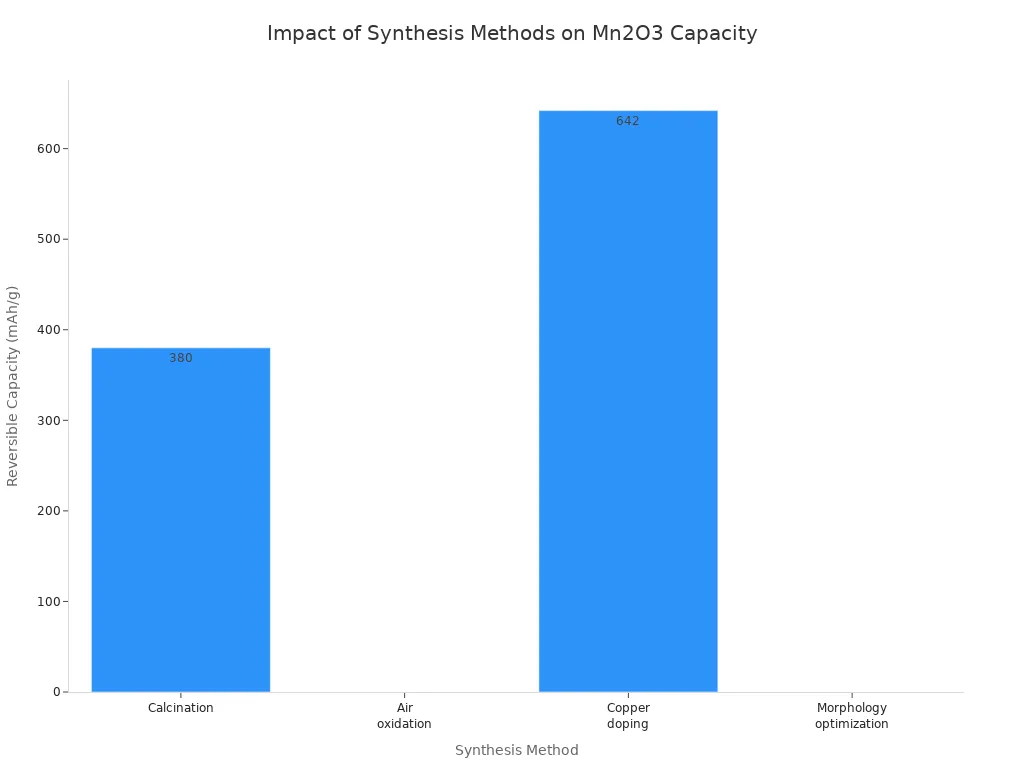
Calcination | Porous structures, nano ribbons | Enhances lithium-ion diffusion and conductivity | Rice straw bundle-like Mn2O3 showed 380 mAh/g reversible capacity after 150 cycles at 200 mA/g current density |
Air oxidation | Uniform particle size, good crystal structure | Simple, low cost method yielding materials with better cycling performance | Produces Mn2O3 with higher discharge capacity and stability compared to commercial samples |
Copper doping | Microspheres | Improves conductivity and lithium-ion diffusion rate | Copper-doped Mn2O3 reached 642 mAh/g after 100 cycles at 100 mA/g current density |
Morphology optimization | Porous microspheres, nano-cones, nano-slices, cubes, spindles | Morphology significantly affects capacity retention and ion transport distance | Unique nano ribbon structure reduces lithium-ion transmission distance, improving capacity retention |
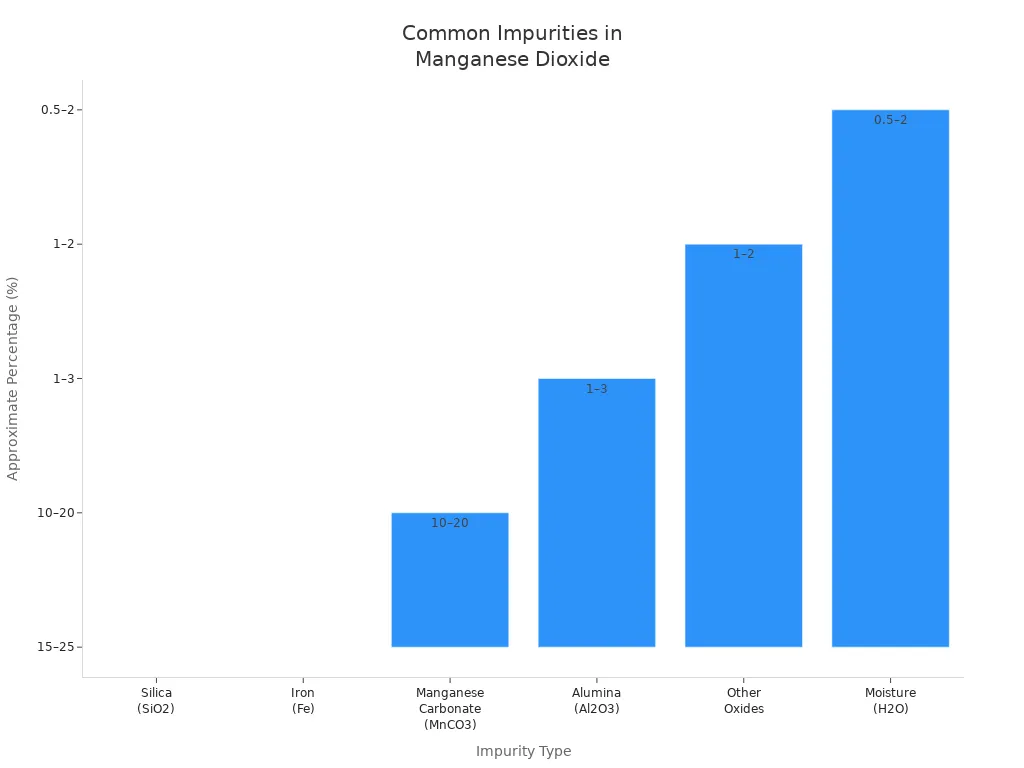
Manganese dioxide can have impurities like silica, iron, manganese carbonate, and alumina. These lower how well the nanomaterials work in imaging and treatment. Cleaning steps, like turning it into manganese(II) nitrate and heating, help remove these extras. Electrolytic manganese dioxide is very pure and good for batteries and imaging.
Making a lot of manganese dioxide at once is hard. Continuous-flow hydrothermal synthesis can make up to 1 kg each day in factories. This green way uses super-hot water and makes very pure material. Wet chemistry methods like hydrothermal, sol-gel, and electrodeposition are fast and cheap. But sometimes the results are not always the same. Chemical vapor deposition gives exact control but only works for small amounts.
Ways to make mno2 keep getting better. Scientists want to find green and cheap ways to help imaging and treatment. Making manganese dioxide and manganese oxide nanomaterials is still very important for batteries, catalysis, and medical imaging.
Advanced Nanostructures and Biosynthesis
Biosynthesis of Manganese Dioxide Nanoparticles
Researchers found green ways to make manganese dioxide nanoparticles. They use plant extracts like lemon and turmeric. This process is safe for the environment. It does not use harmful chemicals. Plant phytochemicals help reduce and stabilize the nanoparticles. Scientists change temperature, pH, and lemon extract ratio to get better results. The best results happen with 75% lemon extract, 50°C temperature, and pH 3.4.
Aspect | Details |
|---|---|
Biosynthesis Method | Green synthesis using lemon extract and curcumin |
Advantages | Environmentally friendly, cost-effective, scalable |
Optimization Technique | Response Surface Methodology (RSM) |
Optimal Conditions | Lemon extract ratio: 75%, Temperature: 50°C, pH: 3.4 |
Characterization Techniques | UV-visible spectroscopy, FTIR, SEM |
Applications | Energy storage, photocatalysis, adsorbents, sensors, biomedical molecule detection |
Environmental Impact | Green synthesis reduces energy consumption and toxic by-products |
Biological Materials Used | Lemon and turmeric extracts |
Biosynthesis makes nanoparticles between 19.8 and 63.9 nm in size. Their shape changes with temperature and pH. Chemically made nanoparticles are more even and pure. Biosynthesis has problems with purity and separation, especially with microbes. Still, biosynthesis helps green nanotechnology and supports the environment.
Aspect | Biosynthesized MnO2 Nanoparticles | Chemically Synthesized MnO2 Nanoparticles |
|---|---|---|
Particle Size | More uniform and homogeneous size distribution | |
Shape | Spherical, variable | Homogeneous and well-defined shapes |
Purity | Challenges in purity and separation | High purity and reproducibility |
Environmental Impact | Ecofriendly, non-toxic, sustainable | Use of toxic chemicals, environmental hazards |
Biosynthesis gives a green and safe way to make manganese dioxide nanoparticles. It lowers toxic waste and helps the environment.
Biosynthesis also helps farming. Studies show these nanoparticles boost antioxidant enzymes in animals. They do not hurt animal growth. These nanoparticles lower mineral waste, which helps the environment. Biosynthesis supports green nanotechnology and protects nature.
Manganese Dioxide Nanosheets
Manganese dioxide nanosheets have special shapes and features. Scientists make nanosheets using two main ways. The top-down way uses ion-exchange and exfoliation to get thin sheets. The bottom-up way builds sheets from molecules using chemical reactions. Both ways control how thick and wide the nanosheets are.
Synthesis Method | Structural Characteristics | Thickness Range | Lateral Dimensions | Functional Properties | Applications |
|---|---|---|---|---|---|
Top-Down Approach | MnO2 nanosheets composed of MnO6 octahedra | ~0.9 nm | <50 nm | High surface-to-volume ratio, enhanced catalytic activity, redox reactivity | Biomedical imaging, drug delivery, biosensing |
Bottom-Up Approach (Reductive) | Uniform nanosheets with controlled morphology | 0.77–1.5 nm | 141–200 nm | Tunable catalytic activity, improved stability, biocompatibility | Photothermal therapy, chemo-dynamic therapy, energy storage, optoelectronics, spintronics |
Bottom-Up Approach (Oxidative) | Thin films of nanosheets with larger lateral size | ~10 nm | 2–5 µm | Large surface area, enhanced optical properties | Solar cells, molecular im |
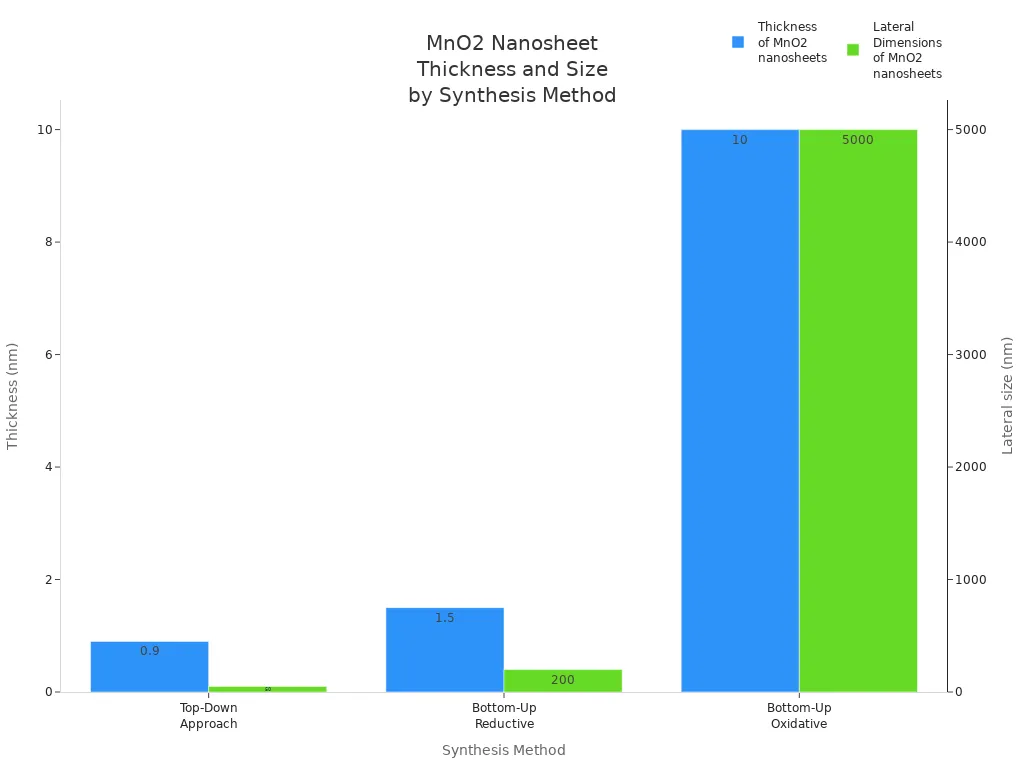
Nanosheets have a high surface-to-volume ratio. This makes them better at catalysis and redox reactions. Manganese dioxide nanosheets glow brightly, so they work well for imaging and biosensing. They are stable and safe for drug delivery and treatments. Scientists use these nanosheets in therapies and energy storage devices.
Synthesis of Manganese Dioxide Nanosheets
Making manganese dioxide nanosheets needs careful control. Top-down methods use ion-exchange and exfoliation. These take longer and make nanosheets with different thicknesses. Bottom-up methods, especially single-step oxidative ones, make pure nanosheets with even thickness. For example, scientists use MnCl2, H2O2, and TMA.OH to make single-layer nanosheets at room temperature. Using KMnO4 and SDS with surfactants makes nanosheets between 0.77 and 0.95 nm thick.
Synthesis Approach | Reaction Materials | Morphology | Lateral Dimensions | Thickness | Key Findings |
|---|---|---|---|---|---|
Top-Down | H0.13MnO2·0.7H2O + TBAOH | Nanosheet structure | <50 nm | ~0.91 nm | Ion-exchange and exfoliation; time-consuming |
Bottom-Up (oxidative) | MnCl2 + H2O2 + TMA.OH | Single-layer sheet | ~200 nm | ~1.5 nm | Single-step synthesis; high purity |
Bottom-Up (reductive) | KMnO4 + SDS | Single-layer nanosheet | ~200 nm | 0.77–0.95 nm | Surfactant-assisted; uniform thickness |
Bottom-Up (oxidative) | MnCl2 + EDTA + NaOH | Thin film nanosheet | 2–5 µm | ~10 nm | Multilayer nanosheets; longer synthesis time |
Bottom-Up (reductive) | KMnO4 + MES | Nanosheet structure | 141 nm | ~1.5 nm | Controlled thickness and mor |
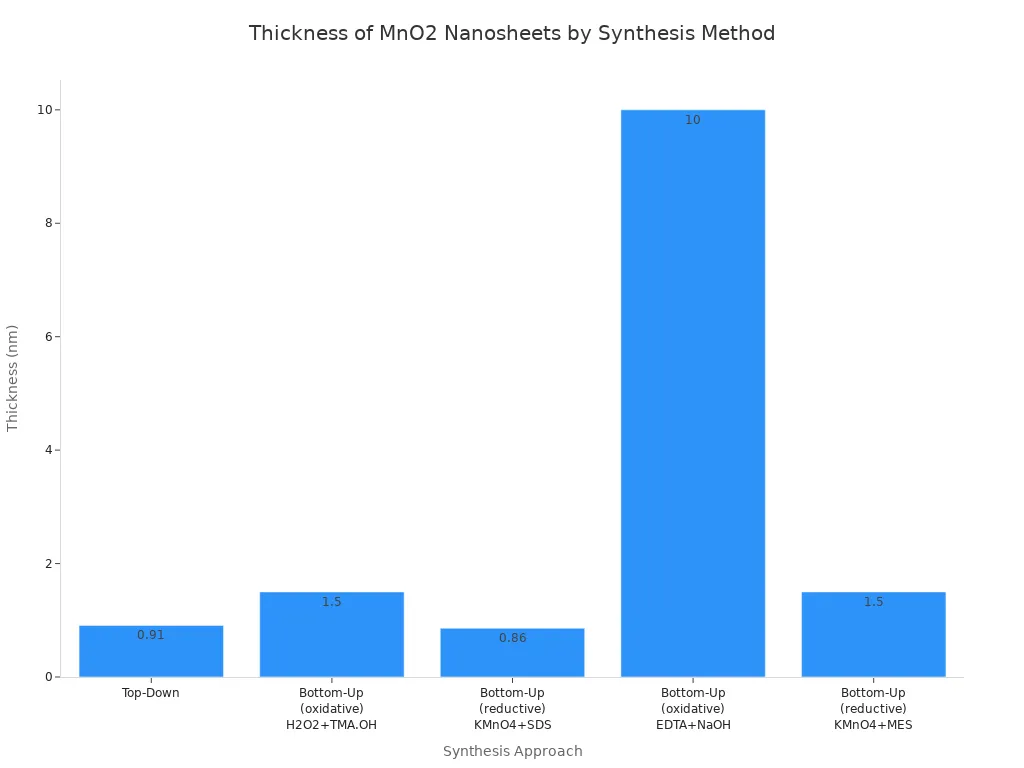
Surfactant type, reaction temperature, and time change the crystallinity and surface area. Surfactant-assisted reactions make very thin nanosheets with both crystal and non-crystal parts. These nanosheets have a large surface area. This helps ions and electrons move faster. During cycling, the structure changes and gets more stable. Making manganese dioxide nanosheets helps with imaging, sensing, and treatments.
Comparative Analysis and Application Matching
Scientists compare ways to make manganese dioxide nanostructures for different uses. Shape, size, and crystallinity affect how well they work in catalysis, energy storage, and imaging. For example, using KMnO4 and glycerol then heating makes porous nano-tablets. Higher heating makes them more crystalline and conductive, which helps performance.
Synthesis Method & Conditions | Resulting MnO2 Nanostructure Morphology | Material Properties | Application Performance & Suitability |
|---|---|---|---|
Gel formation via redox reaction of KMnO4 and glycerol, annealing at 400 °C and 700 °C | Nano-tablet-like porous surface | Higher crystallinity, porous morphology, conductive network | Superior electrochemical performance, high capacitance, suitable for supercapacitor electrodes |
Variation in synthesis parameters | Diverse morphologies: nanoflakes, nanorods, nanowires, nanobelts, nanoflowers, nanosheets | Changes in crystallinity, particle size, surface area, porosity | Morphology and surface area influence conductivity and capacitive behavior |
Researchers use hydrothermal, oxidation-reduction, and biomineralization methods to make good manganese dioxide nanomaterials. These ways are green and safe. Scientists make nanowires, nanospheres, and nanorods to improve stability and reduce changes during charging. Mixing with graphene and carbon nanotubes helps electrons move and raises energy density. Adding transition metals makes them conduct better and react faster. Hybrid structures increase voltage range and power in supercapacitors.
Hydrothermal synthesis is simple and cheap for controlling shape.
Oxidation-reduction methods make nanoparticles without templates.
Biomineralization uses bioorganic substances for green production.
Mixing and doping improve energy storage and imaging.
Scientists change manganese dioxide nanostructures for special uses by controlling how they are made. This helps with imaging, sensing, drug delivery, and treatments.
Recent studies show manganese dioxide clusters on reduced graphene oxide work best for capacitance and charge transfer. This shows how making method and material features matter for supercapacitors and energy storage.
Manganese dioxide nanosheets and nanoparticles are important for imaging, sensing, and treatments. Their large surface area, changeable shape, and safety help drug delivery and cargo loading. Hollow manganese dioxide shapes make cargo loading and treatment better. Making manganese dioxide nanosheets and nanoparticles helps green technology and new solutions for future devices.
Hydrothermal synthesis and biosynthesis are great for making high-purity manganese dioxide. These methods help with imaging, treatment, and green uses. It is important to control ion concentration, phase, and shape. The table below shows which method fits each need. Scientists should think about technical and practical points. This helps with imaging, treatment, green uses, biosynthesis, and fluorescence.
Method | Purity | Imaging | Treatment | Green | Biosynthesis | Fluorescence |
|---|---|---|---|---|---|---|
Hydrothermal | High | ✔️ | ✔️ | ✔️ |
| ✔️ |
High | ✔️ | ✔️ | ✔️ | ✔️ | ✔️ | |
High | ✔️ | ✔️ | ✔️ |
| ✔️ | |
Green | High | ✔️ | ✔️ | ✔️ | ✔️ | ✔️ |
FAQ
What makes manganese dioxide important for imaging applications?
Manganese dioxide is used to make images clearer. Doctors and scientists use it in medical scans. Its special structure helps show more detail in pictures.
How does green synthesis benefit the environment?
Green synthesis uses things from nature instead of harsh chemicals. This keeps the process safer for people and the planet. It also makes less waste, so it is better for the environment.
Can green methods produce high-purity manganese dioxide for imaging?
Green methods can make very pure manganese dioxide. These ways are good for making materials used in imaging. They also help keep the earth safe and support eco-friendly science.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





