Executive Summary
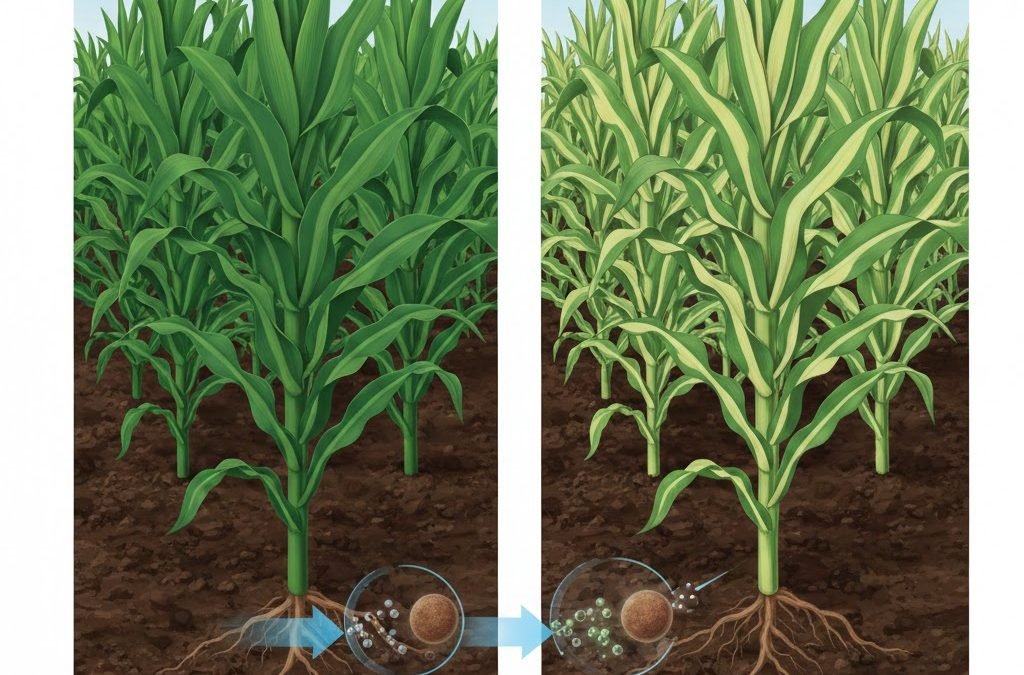
Manganese carbonate (MnCO₃) plays a critical role in correcting manganese (Mn) deficiency in crops, ensuring optimal plant growth, chlorophyll synthesis, and enzyme activation. Manganese deficiency is one of the most common micronutrient limitations in many agricultural soils, particularly in high pH or calcareous conditions where Mn becomes less available. Correcting this deficiency with MnCO₃ can improve photosynthetic efficiency, root development, nitrogen metabolism, and disease resistance—directly impacting key agronomic outcomes such as grain yield, biomass accumulation, and fruit quality. Effective Mn supplementation can increase chlorophyll content by 15–30% and improve crop yields by 10–50%, depending on crop species and deficiency severity. Because MnCO₃ is a stable, slow-release source of manganese, it provides sustained micronutrient availability compared to highly soluble salts, reducing the risk of leaching and phytotoxicity when used properly.
1. Technical Background: What Is MnCO₃ and Why It Matters
1.1 Chemical Nature of Manganese Carbonate
Manganese carbonate (MnCO₃) is an inorganic compound composed of one manganese cation (Mn²⁺) and one carbonate anion (CO₃²⁻). It is a stable, low-solubility manganese mineral with a molecular weight of 114.94 g/mol and a typical purity above 99% in technical agricultural grades.
1.2 Importance of Manganese in Plant Physiology
Manganese is an essential micronutrient involved in several physiological processes:
Photosynthesis: Mn participates in the water-splitting complex of photosystem II, facilitating electron transport and oxygen evolution.
Enzyme Activation: Manganese is a cofactor for enzymes such as Mn-superoxide dismutase (Mn-SOD), arginase, and decarboxylases involved in metabolism.
Nitrogen Assimilation: It supports nitrate reductase activity, influencing nitrogen use efficiency.
Disease Resistance: Mn influences lignin biosynthesis, strengthening plant cell walls against pathogen invasion.
Plants require Mn in trace amounts, typically between 20 and 150 mg/kg dry weight; deficiency occurs when tissue levels fall below this range.
1.3 Manganese Deficiency in Soils
Mn deficiency is most common in:
High pH soils (pH > 7.5) where Mn²⁺ oxidizes to insoluble forms.
Calcareous soils with high calcium carbonate content.
Well-drained sandy soils where Mn is prone to leaching.
Cold, wet conditions that reduce Mn uptake.
Deficiency symptoms include interveinal chlorosis, reduced leaf expansion, and lower root growth.
2. How MnCO₃ Corrects Manganese Deficiency
2.1 Source of Plant-Available Manganese
MnCO₃ corrects deficiency by slowly releasing Mn²⁺ into the soil solution:
MnCO₃ + 2H⁺ → Mn²⁺ + H₂O + CO₂
In acidic microsites (rhizosphere pH 5.5–6.5), MnCO₃ dissolves sufficiently to supply Mn²⁺ without causing toxicity.
2.2 Slow Release vs. Fast Release Sources
Compared to highly soluble sources such as manganese sulfate (MnSO₄·H₂O):
MnCO₃ dissolves slower, providing sustained Mn availability.
Lower risk of salt injury to young roots.
Better suited for pre-plant soil incorporation or base fertilizer blending.
3. Mechanisms & Measurable Benefits in Crops
3.1 Improving Photosynthetic Capacity
Manganese deficiency disrupts the water-splitting complex, reducing chlorophyll production. Correcting deficiency with MnCO₃:
Increases chlorophyll content (SPAD values) by 15–30%.
Enhances photosynthetic electron transport efficiency (Fv/Fm).
This directly supports higher biomass accumulation.
3.2 Root Growth and Nutrient Uptake
Adequate Mn:
Promotes root elongation (root dry weight increases by 10–20% compared to deficient plants).
Improves uptake of other nutrients (e.g., phosphorus uptake enhanced by 8–12%).
3.3 Nitrogen Metabolism
Mn activates nitrate reductase:
Increases nitrate assimilation efficiency.
Reduces nitrate accumulation in leaves, improving plant health and utilization of applied nitrogen.
3.4 Disease Tolerance
Mn contributes to lignin synthesis:
Thickens cell walls.
Enhances resistance to soil-borne fungal pathogens (disease incidence can drop by 15–25% in susceptible cultivars).
4. Application Methods & Best Practices
4.1 Soil Application
Broadcast Incorporation: Mix MnCO₃ (0.5–3 kg/ha Mn equivalent) into topsoil before planting.
Band Placement: Place near seed row for crops sensitive to early deficiency.
Best when soil pH is <7.0 locally; in high pH soils, acidifying amendments (e.g., sulfur) may improve dissolution.
4.2 Foliar Application
MnCO₃ can be formulated into fine suspensions for foliar sprays:
Apply 0.1–0.3% Mn solution at critical growth stages (e.g., V4–V6 in corn).
Ensure leaf wetting and avoid sprays during heat stress to minimize leaf burn.
4.3 Rate Guidelines by Crop
| Crop | Typical Mn Requirement (kg Mn/ha) | Suggested MnCO₃ Rate¹ |
|---|---|---|
| Corn | 1.5–2.5 | 3.0–6.0 kg MnCO₃/ha |
| Soybean | 1.2–2.0 | 2.5–5.0 kg MnCO₃/ha |
| Wheat | 1.0–1.8 | 2.0–4.5 kg MnCO₃/ha |
| Fruit Trees² | 1.8–3.0 | 4.0–7.0 kg MnCO₃/ha |
¹ Adjust based on soil test Mn and pH.
² Apply in split doses for perennial systems.
5. Soil and Tissue Testing: Monitoring Effectiveness
5.1 Soil Testing Standards
DTPA-extractable Mn: Critical sufficiency range often > 5 mg/kg.
Below this, deficiency risk rises significantly.
5.2 Plant Tissue Analysis
Young leaf blades: Sufficiency typically 20–150 mg Mn/kg dry weight.
Interveinal chlorosis plus low tissue Mn confirms deficiency.
Frequent testing (pre-plant and at vegetative stages) guides corrective application timing.
6. Specification Table: Agricultural MnCO₃
| Parameter | Typical Agricultural Grade | Why It Matters |
|---|---|---|
| Mn content (%) | ≥ 30 | Indicates actual Mn supply potential |
| Carbonate (CO₃) (%) | ≥ 55 | Confirms chemical composition |
| Particle Size (D50, µm) | 30–100 | Influences dissolution rate |
| Solubility (g/L @ pH 5.5) | 0.5–1.5 | Availability in rhizosphere |
| Heavy Metals (Fe, Cu, Pb, Cd) (ppm) | < 50 each | Prevents secondary micronutrient issues |
| Moisture (%) | ≤ 1 | Ensures storage stability |
7. Quality Control & Testing Methods
7.1 Chemical Assay
ICP-OES / ICP-MS: Determines Mn, Fe, Cu, Pb, Cd contents.
Ensures fertilizer meets declared specifications.
7.2 Particle Size Analysis
Laser diffraction: Reports D10/D50/D90.
Smaller D50 enhances availability; too fine increases dust and handling issues.
7.3 Solubility Tests
Simulates dissolution under controlled pH.
Helps predict field performance.
7.4 Sampling Protocols
Collect representative samples from multiple bags or a bulk load.
Ensure consistent quality for agronomic outcomes.
8. Practical Considerations for Farmers & Advisors
8.1 Soil pH Management
High pH reduces MnCO₃ dissolution; consider:
Applying sulfur or acidifying fertilizers.
Using localized banding to improve Mn exposure.
8.2 Interaction with Other Nutrients
Excess phosphorus can exacerbate Mn deficiency.
Balanced fertilization enhances overall nutrient uptake.
8.3 Timing and Frequency
Early vegetative stages are critical; delayed correction may not recover yield lost.
9. FAQs
Q1: What soil pH range is ideal for MnCO₃ effectiveness?
A: MnCO₃ is most effective when localized pH is ≤ 7.0; above pH 7.5, availability declines. Soil pH testing must guide management.
Q2: Why not use only manganese sulfate?
A: MnSO₄ is highly soluble and can cause salt injury at high rates. MnCO₃ offers slower release and reduced risk when balanced with crop needs.
Q3: How soon after application will crops respond?
A: Visual improvement may be seen in 7–14 days when deficiency is moderate and conditions favor Mn release.
Q4: Can MnCO₃ be mixed with other fertilizer blends?
A: Yes, but compatibility and particle size should be checked to prevent segregation or reactions.
Q5: What rates should be avoided to prevent toxicity?
A: Avoid exceeding 10 kg Mn/ha total Mn without soil test justification, as excess Mn can interfere with Fe and other micronutrients.
10. Final Practical Checklist for Mn Deficiency Management
Conduct soil and tissue tests before application.
Adjust pH and organic matter to improve Mn availability.
Use MnCO₃ at rates based on soil test recommendations.
Apply early in crop development for best response.
Monitor crops for visual symptoms and follow-up with tissue testing.
Ensure fertilizer quality via certified analysis (Mn content, particle size, heavy metals).
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.