Manganese dioxide thermal stability means manganese (iv) oxide does not change much when heated, especially during calcination. Scientists check this stability with tools like the Turbiscan Lab Expert. This tool shows how well mnox suspensions stay mixed. If it has strong thermal stability, mnox works well as a catalyst. This makes it useful for batteries and industrial reactions.
Industries pick manganese dioxide for energy storage, cleaning the environment, and catalytic uses. Mnox and manganese (iv) oxide keep their shape during calcination. This helps mnox work well as a catalyst in hot catalytic processes, like in batteries and special materials. Catalytic performance depends on doing calcination many times. Mnox acts as a steady catalyst each time.
Mnox-based catalysts are safer and cheaper for energy storage. This is because manganese is easy to find in the earth. Catalysts with mnox also help with green synthesis and cleaning the environment.
Manganese dioxide thermal stability helps mnox stay active through many catalytic cycles, even during calcination. This makes it a great choice for catalysts in new technology.
Manganese dioxide does not break down when heated. It stays strong and works well as a catalyst in batteries and factories. The shape and structure of manganese dioxide help it resist heat. Adding things like tin or cobalt also helps it work better. Different types of manganese dioxide handle heat in different ways. β-MnO2 and α-MnO2 are the best at staying stable in heat. They are good for using many times. Heating can change how manganese dioxide looks and works. This can change how well it does its job. If you heat it carefully, it will stay active longer. Scientists use special tools to test manganese dioxide. They try to make it stronger and better for batteries and catalysts.
Manganese dioxide thermal stability
Definition
Manganese dioxide thermal stability means manganese (iv) oxide keeps working when it gets hot. This is important for mnox, especially during calcination. When heated, manganese (iv) oxide can change in different ways. The decomposition temperature is when the material starts to break down. For manganese (iv) oxide, this usually starts at 460 °C. It can go up to 650 °C, depending on its phase and structure.
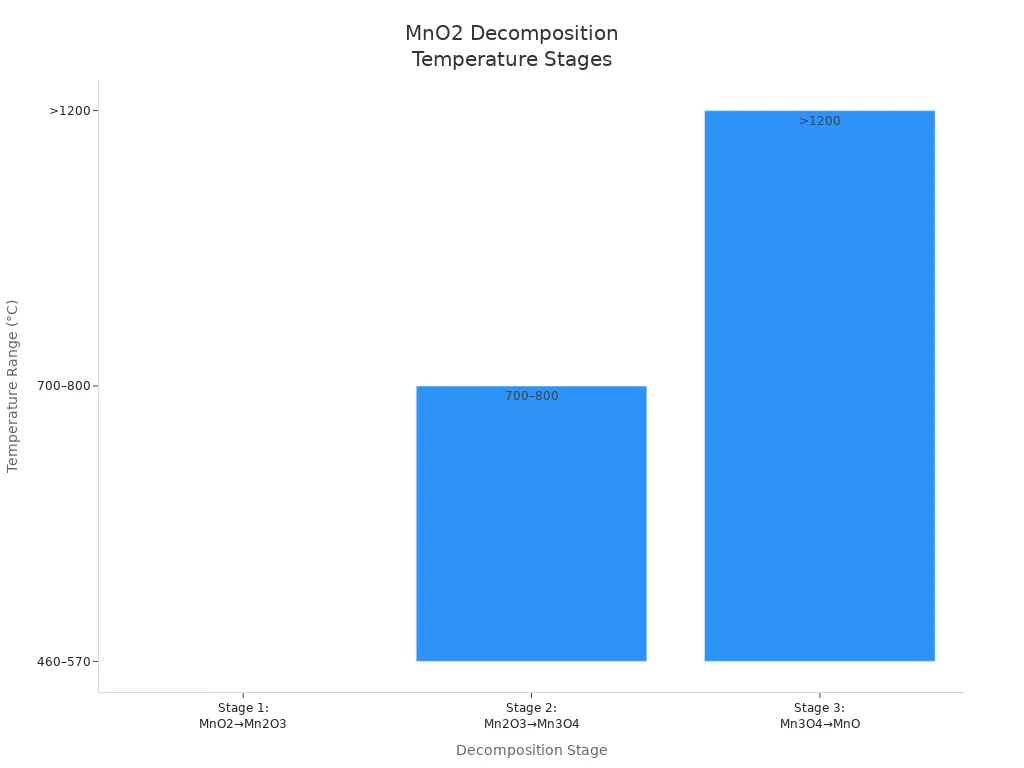
The table below shows the main steps of thermal decomposition for manganese (iv) oxide:
Decomposition Stage | Temperature Range (°C) | Description and Notes |
|---|---|---|
Stage 1 | 460–570 | MnO2 changes to Mn2O3; β-MnO2 finishes changing at about 650 °C, α-MnO2 partly changes and keeps its shape up to 300 °C, showing better resistance to heat |
Stage 2 | 700–800 | Mn2O3 changes more and becomes Mn3O4 |
Stage 3 | >1200 | Mn3O4 turns fully into MnO |
The decomposition temperature depends on the phase of manganese (iv) oxide. For example, α-MnO2 has a tunnel structure that traps molecules. This gives it better thermal stability than β-MnO2. During calcination, β-MnO2 nanorods get thinner from room temperature to 500 °C. α-MnO2 keeps its shape until above 300 °C.
Phase changes happen as the temperature goes up. The main changes are:
Phase Transition | Temperature Range (°C) | Notes |
|---|---|---|
Mn(II) glycolate → Mn3O4 | Organic parts break down and oxidation happens together | |
Mn3O4 → Mn5O8 | 250 – 550 | Oxidation depends on air and heating speed; some direct change to α-Mn2O3 can happen |
Mn5O8 → α-Mn2O3 | Above ~530 (up to 550) | Change happens even when oxygen is present |
These changes affect how mnox works as a catalyst, especially when calcination happens many times.
Influencing factors
Many things affect the thermal stability of mno2. The structure, doping, shape, how it is made, and the environment all matter.
Structure and Morphology: The phase and shape of manganese (iv) oxide decide how well it handles heat. α-MnO2, with its tunnel structure, stays stable during calcination better than β-MnO2. The way it is made can control which phase forms. For example, using KMnO4 makes aggregated α-MnO2, which is the most stable. Other ways can make mixed or less stable phases.
Synthesis Method (Oxidizing Agent)
Resulting MnO2 Phase(s)
Impact on Thermal Stability and Performance
KMnO4
Aggregated α-MnO2
Most stable phase, showing best thermal stability from how it is made
K2S2O8
Mixed α- and γ-MnO2
Middle level stability and performance
(NH4)2S2O8
γ-MnO2 at low temp; β-MnO2 at high temp
Phase change affects stability; β-MnO2 is less stable at high temperatures
Doping: Adding other elements like TiO2, SiO2, or Fe can make manganese (iv) oxide more stable. These help stop sintering and make oxygen move easier. For example, SiO2 slows mass movement at grain edges, while Fe makes Mn–Fe spinels that help oxygen move. TiO2 makes MnTiO3 at high calcination temperature, which keeps the structure strong. The right amount of dopant makes electrochemical performance better and keeps catalytic activity high during calcination.
Environmental Conditions: The air and pressure during calcination change the thermal stability of mno2. In air, manganese (iv) oxide forms layers that protect it and slow more oxidation. The decomposition temperature and phase changes depend on the gas and heating speed. For example, oxidation in air up to 1473 K makes strong oxide layers, which help keep the structure during many calcination cycles.
Note: Thermogravimetry and spectroscopic tools show that the air changes how manganese (iv) oxide acts during calcination. This changes both how it works as a catalyst and how stable it is.
Calcination Temperature and Cycling: The temperature and number of calcination cycles change the structure and performance of mnox. High temperature can cause phase changes and decomposition, but stable phases like α-MnO2 resist these changes better. Doing calcination many times can cause sintering, but adding dopants and having certain shapes help stop this, keeping catalytic and electrochemical performance high.
Catalytic Applications: The thermal stability of mno2 matters for its use as a catalyst. During reactions, mnox needs to keep its structure and work well even after many calcination cycles. Stable phases and doped materials help mnox be a good catalyst in batteries and factories.
Polymorphs
Stability order
Manganese dioxide comes in different forms called polymorphs. The main ones are β, α, γ, δ, and λ. Each one handles heat in its own way, especially when heated during calcination. Scientists found a clear order for how stable they are:
β-MnO2 is the most stable during calcination. It keeps its shape even when very hot.
α-MnO2 is next. It does not change much and still works well as a catalyst after heating.
γ-MnO2 is not as stable. It changes more easily and stops working as a catalyst faster.
δ-MnO2 and λ-MnO2 are the least stable. They change quickly at lower heat and do not last long as catalysts.
Researchers use a method called in situ hydrothermal synthesis to watch these forms appear. First, less stable forms like δ′ and γ′ show up. Then, they turn into more stable forms like α- and β-MnO2. Rietveld refinement and ICP tests check the chemical makeup of α-MnO2. These tests match what scientists expect. Scherrer analysis shows that the size of the particles changes which form appears during calcination. Potassium ion levels also change how long the less stable forms last. This affects how well the catalyst works and what temperature is needed. Knowing the order of stability helps industries pick the best mnox for catalysts and for heating many times.
Phase transitions
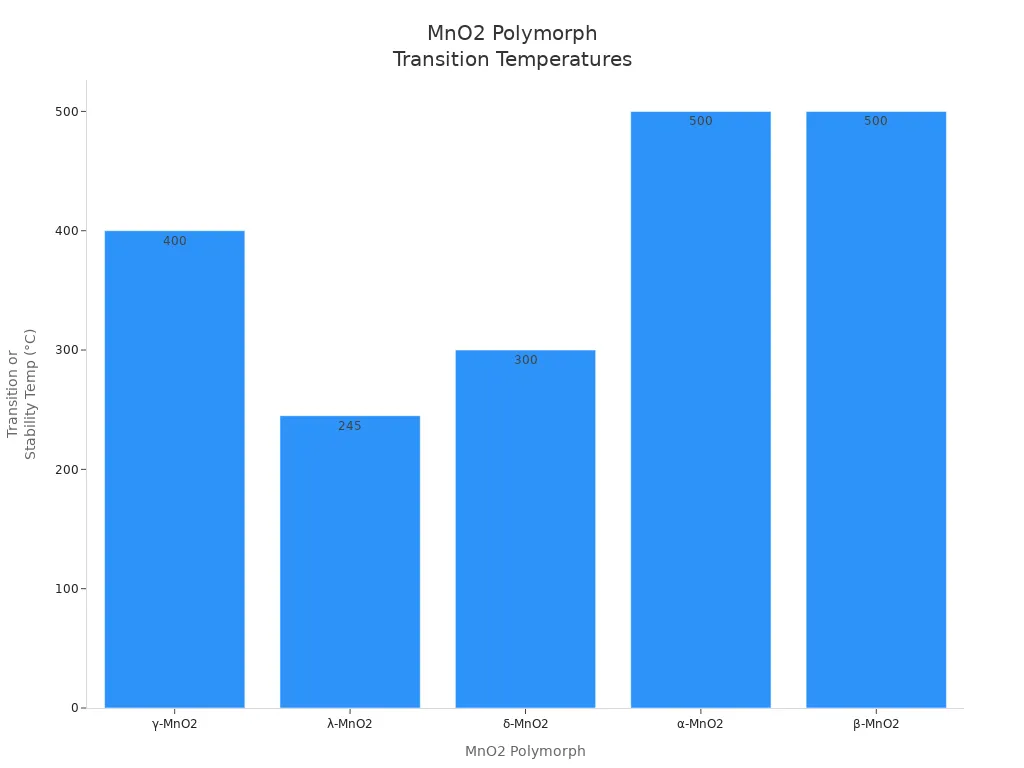
Each polymorph changes at a certain temperature when heated. The table below shows when these changes happen:
MnO2 Polymorph | Phase Transition Temperature Range | Description |
|---|---|---|
γ-MnO2 | Changes to β-MnO2 after relaxing its structure during calcination | |
λ-MnO2 | ~240-250 °C | Changes to β-MnO2, then breaks down to Mn2O3 above this temperature |
δ-MnO2 | >300 °C | Changes to α-MnO2 after losing its layers below 200 °C during calcination |
α-MnO2 | Stable up to 500 °C | Keeps its shape and stays stable when heated |
β-MnO2 | Stable up to 500 °C | Most stable; changes to Mn2O3 above 500 °C because it loses oxygen |
When heated, γ-MnO2 usually turns into β-MnO2. λ-MnO2 and δ-MnO2 lose their shape at lower temperatures, so they are not good for catalysts. α-MnO2 and β-MnO2 keep their shape longer. This makes them better for being used again and again as catalysts. Usually, the breakdown starts when things stick to the mnox surface. Then, electrons move and radicals form, which help the reaction happen. As the temperature goes up, the mnox structure changes. It gets more holes and becomes thicker. These changes affect how well mnox works as a catalyst when it gets very hot.
Heat effects
Structural changes
When mnox is heated, its structure and properties change. Scientists heat it up and see water leave the crystal. First, water on the surface goes away. Then, other water groups inside also leave in steps. If there is a lot of oxygen, more Mn(IV) stays in the structure. This is important for how well mnox works as a catalyst. Taking out water and changing the oxidation state helps make the active phase. This also changes how well mnox works in reactions. For example, γ-MnO2 loses water and turns into β-MnO2. β-MnO2 is more stable when heated. If the temperature gets too high, it changes into Mn2O3, Mn3O4, and MnO. These new forms do not work as well for catalysis.
Heat also changes the surface area and pores of mnox. Heating at about 90 °C makes more tiny pores. This helps mnox take in more things and remove bacteria better. When heated more, the layers stay but the crystal size changes. Smaller crystals have more surface area. This helps mnox work better in reactions. The way heat changes the small structure decides how the active phase forms.
Scientists learned that heating mnox between 250°C and 400°C works best. Mnox can use up to 98% of its Mn(IV) and stays very active as a catalyst.
Doping impact
Adding other elements, called doping, helps mnox stay strong when heated. If scientists add tin or cobalt to α-MnO2, it does not break down until 850–900°C. Without these, it breaks down at 500–550°C. Tin and cobalt go into the tunnels of mnox and make it stronger. They also change how much potassium is inside, which helps with heat stability. Tin and cobalt stop mnox from turning into α-Mn2O3 at high heat. This keeps mnox working longer.
Doped mnox lets electricity move better and works better as a catalyst. Its structure stays strong, even after being heated many times. The kind of dopant, its size, and charge are important. Dopants like Mn4+ help add potassium and make mnox more stable. Some dopants can make holes or twist the structure. This can make mnox less stable and not work as well.
Dopant | Effect on MnO2 | Impact on Catalytic Properties |
|---|---|---|
Tin (Sn) | Makes it break down at higher heat, keeps tunnels strong | Helps mnox work better as a catalyst |
Cobalt (Co) | Makes the structure stronger, stops phase change | Makes mnox more stable and better for reactions |
With the right dopant and heat, mnox is a good choice for catalysts. It keeps its shape, stays active, and works well in reactions.
Applications
Batteries
Manganese dioxide is very important in batteries. Companies use mnox as the main part of the cathode in alkaline batteries. How pure and good mnox is decides how much energy batteries can store. It also affects how long batteries last. If mnox is very pure, batteries give steady power and voltage. This helps devices work longer and more safely. When mnox has strong thermal stability, it keeps its shape during calcination. This lets batteries keep working well for many cycles.
Battery makers care about phase stability too. If mnox changes phase during calcination, batteries lose power and become less safe. Engineers fix this by making the mnox structure better. This helps electricity move and makes batteries last longer. They also add special things to the electrolyte. These form a layer on the cathode. The layer stops manganese from leaving and keeps the battery safe. Sometimes, they add Mn2+ ions to the electrolyte. This cuts down on bad by-products and slows phase changes. All these steps help batteries keep working well and last longer.
Mnox with strong thermal stability helps batteries last longer and work better, even after many cycles and high heat.
Catalytic roles
Factories use mnox as a catalyst in many reactions. Mnox needs to stay stable during calcination to work well as a catalyst. If it does, it keeps its active surface and manganese ions. This helps it work as a porous mno2 catalyst in hot reactions. Mnox is used for co oxidation, VOC oxidation, soot oxidation, and NOx removal. These jobs need catalysts that do not lose their shape after being heated many times.
Mnox is also very good at co oxidation. When mixed with other oxides like ZrO2 and CeO2, it works even better. Zirconium stops the particles from sticking together and helps oxygen move. This makes the porous mno2 catalyst stronger and last longer. In breaking down hydrogen peroxide, mnox with good thermal stability works fast and keeps working after many uses. Making composites with YMn2O5 helps stop phase changes and keeps the catalyst working during heating. These catalysts for co oxidation and other reactions need high heat to get the best phase and stay strong.
Mnox with strong thermal stability and the right calcination temperature gives factories good catalysts for co oxidation and breaking down hydrogen peroxide. These catalysts keep working, hold their shape, and give strong performance.
Analysis methods
Thermal analysis
Scientists use different ways to check how mnox handles heat during calcination. The most used are differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). These tools help scientists see what happens to mnox when it gets hot at different temperatures. DSC checks how much heat mnox takes in or gives off. This shows when mnox changes or reacts during heating. TGA watches how much weight mnox loses as it heats up. This tells when water or other parts leave the mnox. Each time mnox loses weight, it means something is leaving or changing inside. This helps scientists know how mnox breaks down and forms new phases at each temperature.
TGA also gives numbers about how much mnox breaks down. For example, if mnox loses weight between 50°C and 300°C, it means water and some surface parts are leaving. By checking the total weight loss, scientists can see how much mnox is left after heating. This is important for making strong catalysts that last through many heating cycles. In battery studies, DSC and TGA help find dangers like thermal runaway and show how mnox acts at high heat.
Kinetic analysis uses data from DSC and TGA to see how fast mnox breaks down during heating. Scientists use models like Kissinger and Ozawa to find out how much energy is needed and how the reaction works. These models show how mnox changes from β-MnO2 to Mn2O3 and then to Mn3O4 as the temperature goes up. Knowing these steps helps make better catalysts for reactions that need high heat.
Structural tools
Structural tools help scientists see what happens to mnox during and after heating. X-ray diffraction (XRD) is a main tool. XRD shows which forms of mnox are there at each temperature. This helps scientists pick the best form for catalysts. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) let scientists look at the shape and size of mnox pieces. These pictures show how heating changes the surface and structure, which affects how well mnox works.
Other tools, like infrared spectroscopy and Raman spectroscopy, show how chemical bonds change during heating. These tools help scientists see how adding other elements or heating many times changes mnox’s stability. By using these tools, scientists can make mnox catalysts that keep their shape and work well at high heat. This makes mnox a good choice for batteries and factories.
Scientists use both thermal and structural tools to make sure mnox stays strong and active as a catalyst, even after many heating cycles at high temperatures.
Manganese dioxide thermal stability affects how mnox works in factories. Scientists learned that γ-MnO2 with strong MnO6 octahedra and lots of planar oxygen lasts longer as a catalyst. Mnox with special defects and controlled shapes works better. Adding other elements and controlling phases helps catalysts last more cycles. Companies use mnox for catalysts because it stays strong when heated again and again. Mnox catalysts help batteries and chemical plants work well. New technology will use mnox with better structure for advanced catalysts.
Key Finding | Description | Industrial Implication |
|---|---|---|
Crystalline Phase Influence | γ-MnO2 has strong thermal stability and can heal itself, so it lasts longer as a catalyst. | Helps companies pick γ-MnO2 for tough catalytic jobs. |
Doping and Phase Control | Adding nickel makes mnox stronger by keeping crystal sides stable. | Lets engineers make mnox catalysts that last longer. |
FAQ
What is mnox and why is it important?
Mnox stands for manganese oxide materials. Scientists use mnox in batteries and as catalysts. Mnox helps reactions happen faster and makes batteries last longer. Many industries choose mnox because it stays strong when heated.
How does heat affect mnox performance?
Heat can change the structure of mnox. When mnox gets hot, it may lose water or change phase. Stable mnox keeps its shape and works well even after heating. This makes mnox useful for many cycles in batteries and factories.
Why do industries use mnox as catalysts?
Industries use mnox as catalysts because it stays active at high temperatures. Mnox does not break down easily. This means mnox can help with many reactions, like cleaning air or making chemicals, without losing its power.
How do scientists test mnox thermal stability?
Scientists use tools like TGA and DSC to test mnox. These tools show how mnox changes when heated. They measure weight loss and heat flow. This helps scientists pick the best mnox for batteries and catalysts.
Can doping improve mnox stability?
Yes, doping can make mnox stronger. Adding elements like tin or cobalt helps mnox keep its shape at high heat. Doped mnox lasts longer and works better in reactions. This makes doped mnox a good choice for many uses.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





