When MnCO₃ transforms into MnO₂ via calcination for battery use, it undergoes a special heating process called calcination. This process produces MnO₂ with uniform particle size and surface area, which enhances battery performance and longevity. MnO₂ is commonly used in dry cell batteries, including alkaline and zinc–carbon types, as well as in newer battery technologies. The transformation of MnCO₃ into MnO₂ via calcination for battery use helps batteries store more energy and maintain stability through repeated use.
When you heat MnCO₃ in air or oxygen at 370°C to 470°C, it turns into MnO₂. MnO₂ helps batteries store more energy.
You need to control the temperature, time, and oxygen during heating. This makes MnO₂ pure and the right size. This helps batteries work better.
γ-MnO₂ has a special shape and a big surface area. This lets ions move quickly. It helps batteries last longer and have more power.
After heating, you can treat MnO₂ by making it porous or adding metals. This can help batteries last longer and work better.
If you follow careful steps when making and heating MnCO₃, you get strong battery materials. This also helps lower the cost.
MnCO₃ Synthesis
Precipitation Method
You can make manganese carbonate (MnCO₃) by using the precipitation method. This way, you can control how pure it is and how big the particles are. In most labs, people use manganese acetate tetrahydrate (Mn(CH₃COO)₂·4H₂O) and sodium carbonate (Na₂CO₃) as the main chemicals. When you mix these in water, MnCO₃ forms as a solid and sinks to the bottom. In factories, manganese sulfate (MnSO₄) often reacts with sodium carbonate. This also makes MnCO₃ as a solid that comes out of the solution. Some methods use manganese(II) nitrate with ammonia and carbon dioxide to make MnCO₃. You can change the pH, temperature, and how long the reaction lasts to get more product and better quality.
Tip: Add sodium carbonate slowly to the manganese solution. This stops clumps from forming and helps the solid spread out evenly.
Here is a simple chart of the precipitation reaction:
| Reagent 1 | Reagent 2 | Product |
|---|---|---|
| Manganese acetate | Sodium carbonate | MnCO₃ |
| Manganese sulfate | Sodium carbonate | MnCO₃ |
| Manganese(II) nitrate | Ammonia + CO₂ | MnCO₃ |
Preparation Tips
There are some important things to remember for good MnCO₃. Keep the temperature between 25°C and 40°C for the best results. Stir the mixture gently so big particles do not form. Watch the pH and keep it close to neutral. If the pH gets too low, unwanted stuff can show up. Let the reaction go for at least one hour so all the MnCO₃ forms. After the reaction, wash the MnCO₃ with distilled water to get rid of extra chemicals. Dry the MnCO₃ at a low temperature to keep its shape.
- Use clean glassware so nothing else gets in.
- Filter the MnCO₃ carefully to catch small pieces.
- Store the dry MnCO₃ in a closed container to keep out water.
If you follow these steps, you can make MnCO₃ with the right features for batteries.
MnCO₃ Transforms into MnO₂ via Calcination for Battery Use
Calcination Conditions
To make good MnO₂ for batteries, you must watch the calcination process closely. The way MnCO₃ transforms into MnO₂ via calcination for battery use depends on three things: temperature, time, and atmosphere.
Temperature: Set the calcination temperature between 370°C and 470°C. This range lets the right chemical changes happen. It also keeps the material safe from damage.
Duration: How long MnCO₃ stays in the furnace is important. If you heat it for only 1–2 hours, you might get mixed phases. If you heat it for 4 or 5 hours, you get purer MnO₂ or other manganese oxides.
Atmosphere: You need lots of oxygen, like air or pure O₂. Oxygen helps MnCO₃ turn into higher oxidation state oxides like MnO₂. If you use a gas with no oxygen, you will not get the right product for batteries.
Tip: Always use a furnace with good airflow and check the temperature often. Oxygen flow helps you get the best results when MnCO₃ transforms into MnO₂ via calcination for battery use.
The table below shows the main calcination conditions:
Parameter | Typical Range | Effect on Product |
|---|---|---|
Temperature | 370°C – 470°C | Controls phase and purity |
Duration | 1 – 4 hours | Longer time = purer, larger particles |
Atmosphere | Air or O₂ | Needed for full oxidation to MnO₂ |
Each factor affects how MnCO₃ transforms into MnO₂ via calcination for battery use. By changing these settings, you can control the phase, size, and purity of your MnO₂.
Transformation Mechanism
The way MnCO₃ transforms into MnO₂ via calcination for battery use happens in steps. First, dehydration takes place, then more chemical changes happen until you get the final product.
Dehydration (50°C – 300°C): Water leaves the surface and inside the MnCO₃ particles. Heat breaks weak bonds and lets water vapor escape.
Initial Decomposition (200°C – 400°C): MnCO₃ breaks down into manganese(II) oxide (MnO) and carbon dioxide gas. This is the first big change in the material.
Formation of Intermediate Oxides: As you keep heating, MnO turns into other manganese oxides. You might see Mn₃O₄ and Mn₅O₈ form, especially with oxygen. These phases depend on both temperature and how long you heat the sample.
Oxidation to MnO₂ (450°C – 500°C): In an oxygen-rich atmosphere, MnO and other lower oxides gain more oxygen atoms and become MnO₂. This is the key step when MnCO₃ transforms into MnO₂ via calcination for battery use.
Further Transformations (Above 500°C): If you heat above 500°C, MnO₂ can turn into α-Mn₂O₃ or even Mn₃O₄. You should avoid these higher temperatures if you want pure MnO₂ for batteries.
The process also changes the size and shape of the particles. Shorter calcination times give smaller, less dense particles. Longer times make the particles bigger and closer together. You can see these changes with a microscope. For example, Mn₃O₄ nanoparticles can be under 10 nm, but Mn₅O₈ particles can reach up to 30 nm after longer heating in oxygen.
Note: Oxygen not only helps with oxidation but also makes particles larger and more even. This matters because battery performance depends on both the phase and the structure of MnO₂.
You can check if MnCO₃ transforms into MnO₂ via calcination for battery use by using X-ray diffraction (XRD) to see the crystal structure, thermogravimetric analysis (TGA) to track weight loss, and chemical analysis to measure purity.
If you follow these steps, you will get MnO₂ with the right phase and structure for batteries. The process where MnCO₃ transforms into MnO₂ via calcination for battery use lets you control the material’s properties, which is important for making better batteries.
MnO₂ for Batteries

γ-MnO₂ Properties
γ-MnO₂ is special because of its crystal structure. It has both 2×1 and 1×1 tunnels. These tunnels help ions move fast in batteries. The MnO₆ chains in γ-MnO₂ are twisted. This makes the tunnels deeper than in other manganese oxides. The tunnel network helps electricity flow well. This is good when you want to add more active material to a battery.
γ-MnO₂ has many random defects inside. Some are called De Wolff defects and microtwinning. These defects cause packing problems and change how ions move in and out. The high disorder in γ-MnO₂ helps lithium fit inside better. This makes the battery work better. You can use heat to change these features. This can make the material conduct better and last longer.
γ-MnO₂ has a much bigger surface area than other MnO₂ types. This means there are more places for reactions to happen. The table below shows how γ-MnO₂ compares to other types:
MnO₂ Polymorph | Surface Area (m²/g) |
|---|---|
γ-MnO₂ | |
Birnessite | 34 |
Cryptomelane | 23 |
Pyrolusite | 0.17 |
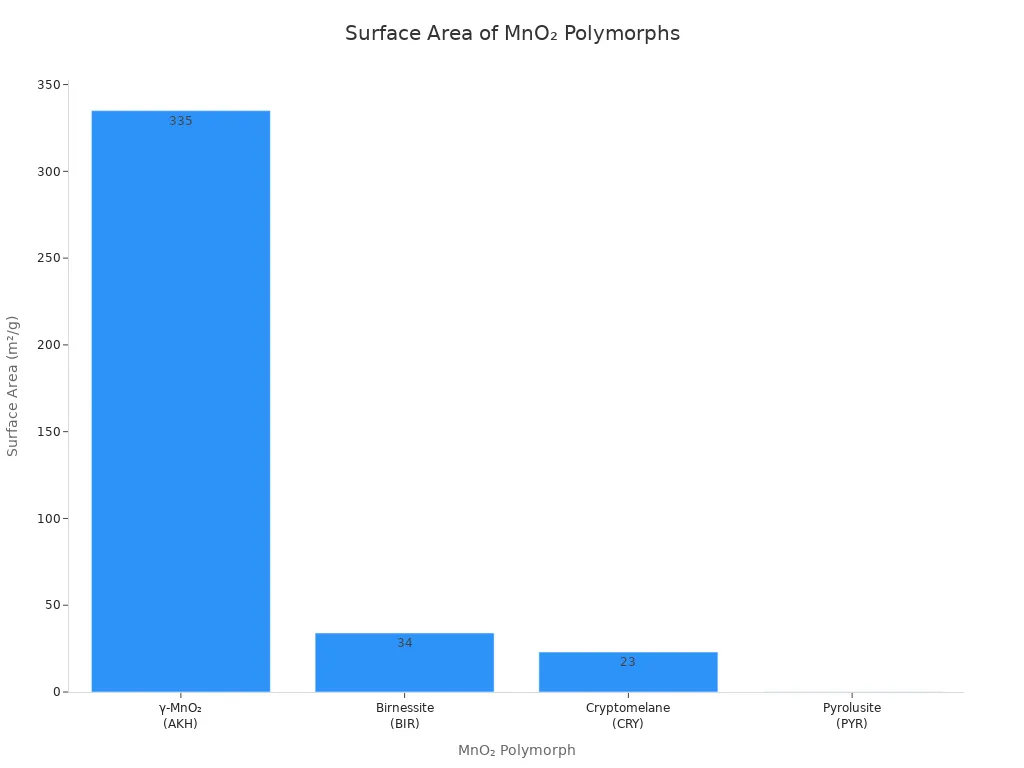
Tip: A bigger surface area helps batteries work better. More ions can react at the same time.
Cathode Performance
γ-MnO₂ works well as a cathode in batteries. It gives high energy density. The best possible capacity is about 308 mAh g⁻¹. In real batteries, γ-MnO₂ gives about 245–250 mAh g⁻¹. This is about 80% of the best value. The difference happens because the structure changes during use. These changes make it hard to recharge the battery many times.
You can make MnO₂ work better with special treatments after calcination. Making it porous or into nano ribbons gives more surface area. This helps the battery last longer. Adding metals like copper makes it conduct better. It also helps lithium ions move faster. Changing the calcination temperature can keep the structure stable. It also gives more places for reactions.
Post-Calcination Treatment | Effect on Battery Performance |
|---|---|
Porous/Nano Morphology | Higher surface area, better cycling stability |
Metal Doping | Improved conductivity, higher capacity |
Temperature Control | Stable structure, more active sites |
Note: Oxygen vacancies made after calcination can help the battery hold more charge. They also help the battery charge and discharge better.
γ-MnO₂ has a special structure and large surface area. This helps it work well as a battery cathode. With the right treatments, it gets even better for storing energy.
You can make good battery materials by doing three main things. First, you need to synthesize MnCO₃. Next, you must use careful calcination. Last, you use the MnO₂ you made in batteries. When MnCO₃ transforms into MnO₂ via calcination for battery use, you get some big benefits:
Nano-structured MnO₂ gives high capacity and lasts many cycles.
γ-MnO₂ cathodes let batteries run longer than older materials.
This process costs less than using cobalt-based materials.
To get better results, control how you make the materials. Check the purity often. Try new oxidizing atmospheres too. Doing these things helps you make better battery materials for future energy needs.
FAQ
What is calcination?
Calcination means heating a material to a high temperature in air or oxygen. You use this process to change the chemical structure. For MnCO₃, you heat it to make MnO₂, which works well in batteries.
Why does MnO₂ work well in batteries?
You get high energy storage and long battery life with MnO₂. Its structure lets ions move quickly. This helps batteries charge and discharge faster.
How do you check if MnCO₃ turned into MnO₂?
You can use X-ray diffraction (XRD) to see the crystal structure. Thermogravimetric analysis (TGA) shows weight changes. These tests help you confirm the right phase formed.
Can you reuse MnO₂ from old batteries?
You can recycle MnO₂ from used batteries. First, you clean and process the material. Then, you check its purity before using it again in new batteries.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.