The α β γ δ manganese dioxide phases have clear differences in their crystal structure. α-MnO2 has wide tunnels. β-MnO2 has narrow tunnels. γ-MnO2 has a mix of tunnel types. δ-MnO2 forms layers. These features affect how electrodes move charge, do redox reactions, and store energy. Tunnel or layered shapes change how well the electrode works. They also affect how much charge it can hold. δ-MnO2’s layers help ions move better. This gives higher energy storage and stable charging. α-MnO2’s big tunnels help charge move faster. This improves energy storage and performance. Each phase’s structure changes redox activity, charge transfer, and energy storage. So, picking the right phase is important for electrode performance and charge storage.
- Manganese dioxide has four main phases. These are α, β, γ, and δ. Each phase has a different crystal shape. The crystal shape changes how well they store charge. It also affects how ions move inside them.
- α-MnO2 has wide tunnels. δ-MnO2 has a layered structure. These features help ions move faster. Faster ion movement means more energy storage. It also means better electrode performance.
- Picking the right manganese dioxide phase is important. It helps batteries and supercapacitors work better. You need to balance charge capacity, stability, and ion movement. This makes devices more efficient.
Manganese Dioxide Crystal Structures
What Are Crystal Structures?
Crystal structures show how atoms are arranged in solids. In manganese dioxide, the main part is the MnO6 octahedron. These octahedra join by sharing edges or corners. This makes two main shapes: tunnel and layered. Tunnel structures have chains of MnO6 octahedra. These chains make open spaces called tunnels inside the crystal. Tunnels can hold water and cations. Layered structures, called phyllomanganates, stack sheets of MnO6 octahedra. Spaces between layers can also hold water and cations. Manganese dioxide can have different manganese valence states. These states can change the shape of the structure. Scientists use X-ray diffraction and electron microscopy to study these structures. The Crystallography Open Database has important data on manganese dioxide phases.
Main features of manganese dioxide crystal structures:
MnO6 octahedra are the building blocks
Tunnel or layered frameworks
Spaces for cations and water
Many manganese valence states
Lots of structural diversity and adaptability
Why Structure Matters
The structure of manganese dioxide changes its properties. For example, β-MnO2 has a rutile structure with narrow tunnels. α-MnO2 has wider channels. These differences affect how ions move in the electrode. Layered δ-MnO2 lets ions move easily between layers. This increases capacitance. Tunnel structures, like in α-MnO2, can store more cations. This improves capacitance and energy storage. Scientists can control how these structures are made. This helps design electrodes that work better. Controlled synthesis makes manganese dioxide with the right structure. This gives high capacitance and fast ion transport. The many manganese dioxide structures give it an edge over other transition metal oxides. This flexibility makes manganese dioxide great for electrodes in batteries and supercapacitors.
Tip: Making manganese dioxide with the right structure helps electrodes store more charge and last longer.
α β γ δ Manganese Dioxide Phases
α-MnO₂ Structure
α-MnO₂ is a tunnel manganese dioxide phase. Its crystal structure has wide tunnels. These tunnels are made by linking MnO6 octahedra in a tetragonal way. The tunnels are about 4.6 Å wide. This lets large cations and water move through. Wide tunnels help the electrode store more charge. They also make ion movement easier. This phase gives high capacitance and fast redox reactions. α-MnO₂ is popular for battery and supercapacitor electrodes. The structure stays stable at high temperatures. This makes it useful for energy storage devices. Hydrothermal and solvothermal methods make α-MnO₂ with nanoneedle or nanowire shapes. These shapes boost electrochemical performance.
β-MnO₂ Structure
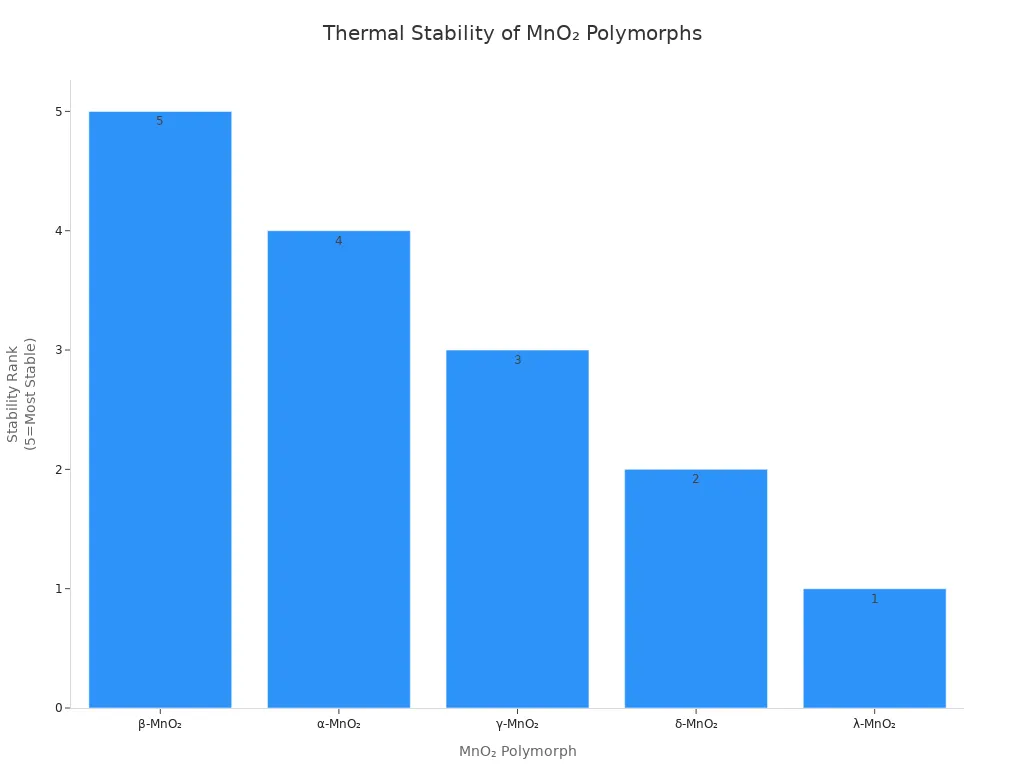
β-MnO₂ is the most stable manganese dioxide phase. Its crystal structure has narrow tunnels. MnO6 octahedra connect in a rutile-type tetragonal pattern. The tunnels are about 1.89 Å wide. This is smaller than hydrated sodium ions. Small tunnels limit cation and water movement. This lowers the electrode’s capacitance and charge storage. β-MnO₂ has high crystallinity. It forms single crystalline nanorods with clear lattice lines. The phase stays stable up to 500 °C in air. It is the strongest manganese dioxide crystal phase.
Structural Feature | Description / Value |
|---|---|
Crystal phase | Tetragonal, space group P42/mnm |
Tunnel structure | 1 × 1 tunnels (one-dimensional channels formed by corner-sharing MnO6 octahedra) |
Tunnel size | Approximately 2.3 Å × 2.3 Å (some reports 1.89 Å) |
Tunnel density | About 0.104 Å⁻², with two tunnels per formula unit |
Morphology | Nanorods with square cross section (~20 nm side length), lengths extend to micrometers |
Crystallinity | Single crystalline nanorods confirmed by SAED patterns |
Electrochemical implication | Small tunnel size limits cation accommodation, leading to poorer electrochemical performance |
β-MnO₂’s dense structure and small cell make it very stable. The phase keeps its shape after many charge and discharge cycles. There is only a little lattice expansion. This stability makes β-MnO₂ good for long-lasting uses. But its small tunnels limit capacitance and redox activity.
γ-MnO₂ Structure
γ-MnO₂ has a mixed tunnel structure. Its crystal phase has both (2 × 1) and (1 × 1) tunnels. These tunnels form an orthorhombic symmetry. The (2 × 1) tunnels are bigger than those in β-MnO₂. They are smaller than those in α-MnO₂. This special setup gives γ-MnO₂ middle-level electrochemical properties. The phase often has defects like cation vacancies and reduced manganese ions. It also has structural water. Defects show up at boundaries between ramsdellite and pyrolusite domains. They also appear near cation vacancies.
Cation vacancies and reduced manganese ions (Mn³⁺)
Structural water molecules
Defect areas at crystal boundaries
Higher energy at defect spots, causing Mn⁴⁺ reduction at higher voltages
Lattice expansion and strain from Jahn–Teller distortion
Mechanical strain from repeated charging and discharging
Permanent lattice expansion and bending of the oxygen framework
These defects change the electrode’s redox behavior and charge transfer. They also affect cycling durability. The phase turns into β-MnO₂ at high temperatures. It is less stable than α- or β-MnO₂. Defects raise capacitance but can lower rechargeability and stability.
δ-MnO₂ Structure
δ-MnO₂ is different because it has a layered structure. MnO6 octahedra stack in sheets. This makes spaces between layers about 7 Å wide. These spaces hold cations like K+ and water. The cations and water act as pillars and keep the structure stable. The layers let ions move easily between sheets. This helps charge storage and capacitance.
δ-MnO₂ loses its layered order below 200 °C. It changes into α-MnO₂ above 300 °C. This makes it less stable than tunnel phases. Interlayer cations and water help keep δ-MnO₂ stable. They also improve electrochemical performance. FTIR finds structural water. Thermogravimetric analysis measures bound water. These interlayer parts lower charge transfer resistance. They also boost cyclic stability. δ-MnO₂ electrodes have high capacitance and fast redox reactions. Ions move quickly through the layered structure.
The change from δ-MnO₂’s layers to tunnel phases involves shearing and bending of the MnO2 layers. This breaks the Mn–O framework and makes Mn3+ ions. This shows the main difference between layered and tunnel manganese dioxide phases.
Note: Interlayer cations and water in δ-MnO₂ help ions move, improve electron transport, and make electrodes work better.
Comparison of Phases
Key Differences
Manganese dioxide has many crystal structures. Each phase is arranged in a special way. This changes how electrodes work and hold charge. The α, β, γ, and δ phases have different tunnel sizes and layers. The δ phase makes flat layers. Potassium ions and water sit between these layers. This changes the space between them, which is about 0.67 nanometers. The layers are different from the tunnels in α, β, and γ phases.
The β phase has the smallest tunnels. They are about 0.23 nanometers wide. This phase is very stable and has low energy. The γ phase is in the middle. It has mixed tunnel sizes and more disorder. The α phase has bigger tunnels, about 0.46 nanometers wide. Potassium ions help keep these tunnels stable. The amount of potassium used when making the material changes which phase forms. Small particles and less potassium make the δ phase. More potassium helps the α phase stay stable.
Physical properties are also different for each phase. The δ phase has the biggest surface area and pore volume. This helps ions move and stick quickly. It makes the electrode work better. The α phase has big tunnels and a medium surface area. The β phase has small tunnels. This means less space for ions and a smaller surface area. The γ phase has tunnels of different sizes and many defects. These change how porous it is and how well it forms crystals.
The crystal structure of each manganese dioxide phase controls how ions move, how much charge the electrode can hold, and how stable the material is when used many times.
Tunnel vs. Layered Structures
Tunnel and layered shapes are different in manganese dioxide. Tunnel phases like α, β, and γ have three-dimensional shapes. The α phase has wide tunnels. Big ions and water can move easily through them. Potassium ions inside the tunnels help keep the structure strong and help ions move. The β phase has narrow tunnels. This makes it hard for ions to move and lowers how much charge it can hold. The γ phase mixes tunnel sizes and has more defects. This can help hold more charge but makes it less stable.
Layered structures, like the δ phase, stack sheets together. Potassium ions and water sit between the layers like pillars. This gives the δ phase the biggest surface area and pore volume. Ions move fast between the layers. This helps the electrode work better and hold more charge. The δ phase is good for supercapacitors and batteries. It lets ions move quickly and stores lots of charge.
Comparing tunnel and layered shapes shows differences in how they work. Tunnel phases are usually more stable, especially the β phase. But they may not hold as much charge. Layered phases like δ manganese dioxide hold more charge and work faster. But they can have problems like swelling and losing their shape after many uses.
The table below shows the main differences in structure and properties for the four manganese dioxide phases:
MnO2 Phase | Structure Type | Tunnel/Layer Size (nm) | Interlayer Features / Spacing (nm) | Crystallinity Notes | Surface Area & Porosity Characteristics | Electrochemical Performance | Suitability for Supercapacitors |
|---|---|---|---|---|---|---|---|
β-MnO2 | 3D tunnel (1×1) | 0.23 × 0.23 | N/A | Most stable; narrow tunnels | Low surface area and porosity | Lower specific capacitance | Limited |
α-MnO2 | 3D tunnel (2×2) | 0.46 × 0.46 | Stabilized by K+ ions | Larger tunnels; stabilized by potassium | Moderate surface area; good adsorption | High specific capacitance | Good |
γ-MnO2 | 3D tunnel (1×2, 1×1) | 0.23 × 0.46 | N/A | Disordered; many defects and vacancies | Variable porosity; affected by defects | Moderate capacitance | Moderate |
δ-MnO2 | 2D layered | N/A | ~0.67 (interlayer spacing) | Layered; interlayer K+ and water; variable order | Highest surface area and pore volume | Highest specific capacitance | Excellent |
Tunnel structures in manganese dioxide change how ions move. Potassium ions inside the tunnels of α phase nanowires make the tunnels bigger. This helps lithium ions move faster. It also improves how much charge the electrode can give and how fast it works. If you remove potassium, these benefits go away. How the tunnels are ordered also matters. Straight tunnels help ions move quickly. Mixed tunnels slow them down.
Layered manganese dioxide phases have some problems with ion movement. Ions move slowly, and the material does not conduct ions well. The layers can break down after many uses. Tunnel phases, especially those with potassium, let ions move faster and stay stable longer.
Electrochemical Performance and Applications
Performance in Batteries
The way manganese dioxide is built affects how it works in batteries. α-MnO2 and δ-MnO2 are the best for batteries. They have wide tunnels and layers. These help ions move fast. Fast ion movement helps charging and discharging. The electrode can hold more charge. It keeps high capacitance. In batteries, α-MnO2 lets Zn2+ ions go into its tunnels. This gives high capacity and stays stable for a long time. δ-MnO2 has layers with big spaces. These layers let H+ and Zn2+ ions move in and out. The electrode starts with high capacitance. But weak bonds between layers can make it less stable later.
MnO2 Polymorph | Structure Type | Key Structural Features | Impact on Battery Performance |
|---|---|---|---|
α-MnO2 | Tunnel structure | Larger tunnel size, higher surface area | Facilitates Zn2+ ion insertion and diffusion, resulting in high capacity and good long-term stability |
δ-MnO2 | Layered structure | Two-dimensional layers with broad interlayer spacing (~0.7 nm) | Enables efficient intercalation/deintercalation of H+/Zn2+ ions, leading to high initial capacity but lower stability due to weak interlayer bonding |
β-MnO2 | Rutile-type tunnel | Dense structure with narrow 1×1 tunnels (~1.89 Å) | Restricts ion movement mainly to small ions (H+), limiting discharge capacity and overall electrochemical performance |
γ-MnO2 | Mixed tunnel | Disordered tunnels, more defects | Moderate performance, less stability |
The table shows how the structure changes battery results. α-MnO2 and δ-MnO2 are best for supercapacitors and batteries. Their shapes help ions move fast and hold lots of charge. β-MnO2 and γ-MnO2 do not work as well. Their tunnels are too small or mixed up.
MnO2 Phase / Substrate | Specific Capacitance (F g⁻¹) | Capacitance Retention (%) after 1000 cycles | Notes |
|---|---|---|---|
MnO2/Ag | 89 | Highest stability and capacitance; strong adhesion; best conductivity | |
MnO2/Ni | 150 | 75 | Moderate stability and capacitance |
MnO2/Al | 101 | 65 | Lowest stability and capacitance |
Tip: Manganese dioxide electrodes with wide tunnels or layers work best in batteries and supercapacitors.
Catalytic and Environmental Uses
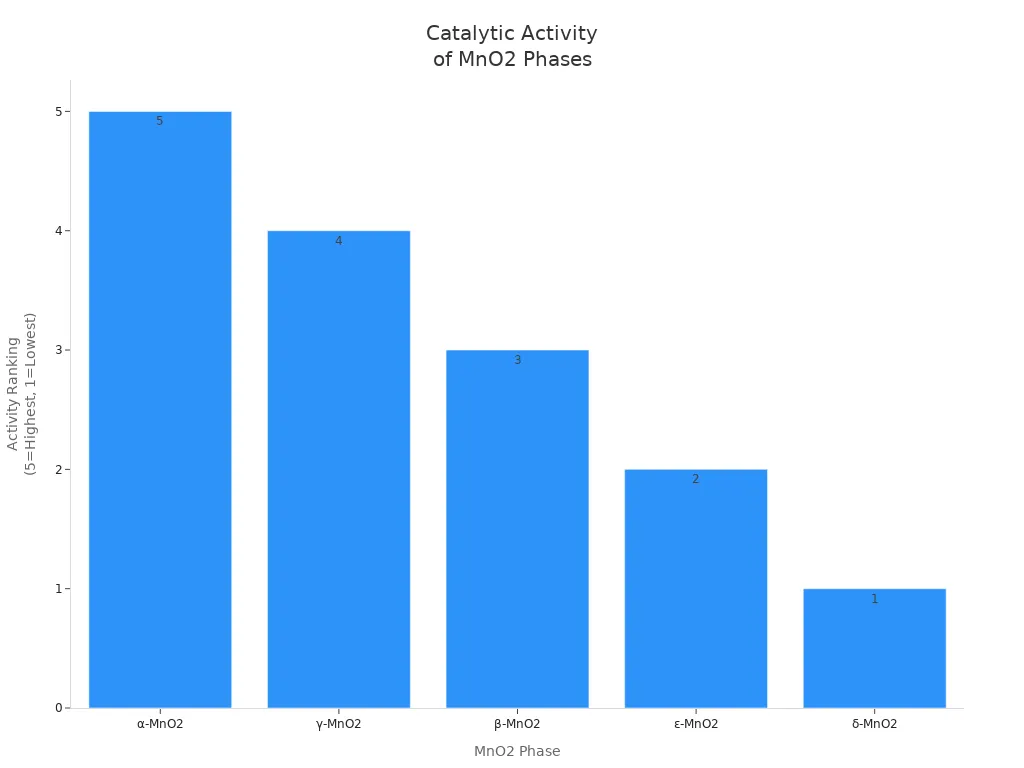
Manganese dioxide helps speed up many chemical reactions. The α phase is the best catalyst. Its structure and lots of Mn(IV) help electrons move. γ-MnO2 is also a good catalyst. β-MnO2 works okay. δ-MnO2 is the weakest catalyst. Its shape does not help electrons move fast.
Manganese dioxide is used to clean pollution and help start fires. β-MnO2 lowers the temperature needed to burn wood. It gives off oxygen when burning. This helps the fire. Scientists use manganese dioxide to clean dirty water. Tiny living things help remove manganese by biosorption and oxidation. These ways do not make toxic waste. They are good for cleaning the environment.
New ways to make manganese dioxide let scientists change its structure. They use thin films, atom doping, and defect engineering. These methods make it work better in batteries and as a catalyst. Supercapacitors use these new materials to store more charge and last longer.
Manganese dioxide has different crystal shapes in each phase. The α phase has wide tunnels. This helps the electrode work better and hold more charge. β manganese dioxide has narrow tunnels. These small tunnels make it hard for ions to move and store less charge. γ manganese dioxide has both big and small tunnels. This gives it average performance. δ manganese dioxide forms layers instead of tunnels. These layers help supercapacitors store the most charge. Picking the right phase makes electrodes work better, hold more charge, and last longer.
MnO2 Phase | Structure Type | Capacitance Impact | Electrode Performance |
|---|---|---|---|
α | Wide tunnels | High specific capacitance | Excellent |
β | Narrow tunnels | Low specific capacitance | Limited |
γ | Mixed tunnels | Moderate specific capacitance | Moderate |
δ | Layered | Highest specific capacitance | Outstanding |
Pick manganese dioxide by looking at the electrode’s shape, how much charge it can hold, and how well it works.
How you make manganese dioxide and its shape can change how well supercapacitors work and how much charge they store.
FAQ
What makes manganese dioxide phases different in energy storage?
Each phase has its own way of arranging manganese and dioxide. Some have tunnels, and some have layers. These shapes change how ions can move. This movement affects how much energy the material can store.
Why does capacitance vary between manganese dioxide phases?
Capacitance changes because of tunnel size, layer space, and how manganese atoms join together. Bigger tunnels or wider layers let more ions pass through. This helps the material hold more charge.
How can someone choose the best manganese dioxide phase for high capacitance?
They should check the structure first. Manganese dioxide with wide tunnels or layers stores more charge. The best choice is a stable phase with well-arranged manganese atoms.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





