Manganese monoxide used in battery manufacturing is transforming how we think about batteries in 2025. This form of manganese is found in new electrodes, helping batteries charge faster and more efficiently. Manganese dioxide plays a crucial role in maintaining steady lithium-ion movement, which extends battery life. Additionally, manganese helps keep batteries safe by preventing them from overheating. The strength of the cathode is enhanced by manganese dioxide, allowing batteries to endure more charge cycles. Incorporating manganese monoxide used in battery manufacturing also helps reduce costs, making batteries more affordable. Overall, manganese monoxide used in battery manufacturing boosts energy capacity and improves battery performance, while manganese dioxide ensures batteries remain durable and reliable.
Manganese monoxide helps batteries work faster and safer. It also helps batteries store more energy by making the electrode design better. It keeps lithium-ion movement steady. Batteries with manganese last longer. They also charge faster. This makes them good for electric cars, electronics, and energy storage. Using manganese makes batteries cost less. It also helps the environment because mining and recycling are easier. New ideas with manganese oxide composites and new battery types make batteries work even better. They also help batteries last longer. Top companies use manganese-based batteries now. They do this to meet the need for safer, smaller, and better energy solutions.
Manganese Monoxide Used in Battery Manufacturing
Advanced Electrodes
Big changes happen in electrode design with manganese monoxide. Scientists add surfactants to the electrolyte. This helps form neat liquid crystal shapes on the electrode. These shapes guide how manganese oxide crystals grow. The crystals become better at carrying electricity. This means ions move faster and there are fewer bad reactions. Batteries can store more energy and last longer.
Both manganese dioxide and manganese monoxide help make these new electrodes. Zinc and manganese dioxide can join together to make temporary electrodes when charging. They break apart when the battery is used. This way, batteries can store much more energy. They also last almost four times longer than old zinc-manganese dioxide batteries. Problems like dendrites and corrosion are less likely. Batteries can be recharged thousands of times. This makes manganese batteries good for storing lots of energy for the power grid.
Note: Many patents talk about manganese oxide electrodes, especially with manganese dioxide and carbon nanostructures. Patents often mention manganese dioxide but not much about manganese monoxide. Still, manganese dioxide is very important for new battery designs.
Lithium-Ion Movement
Manganese monoxide changes how lithium ions move in batteries. When charging, manganese ions leave the cathode and move into the electrolyte. This changes the chemicals near the electrodes. It also changes how lithium ions travel inside the battery.
Here is a summary of test results showing what happens:
Parameter | Observation / Result |
|---|---|
Measurement technique | In-situ 1H MRI imaging of Li/LMO cell during charge-discharge cycles |
Signal intensity near cathode | Goes up during charging, showing Mn2+ moves into the electrolyte |
Effect on solvent protons | Mn2+ changes the MRI signal and makes it stronger |
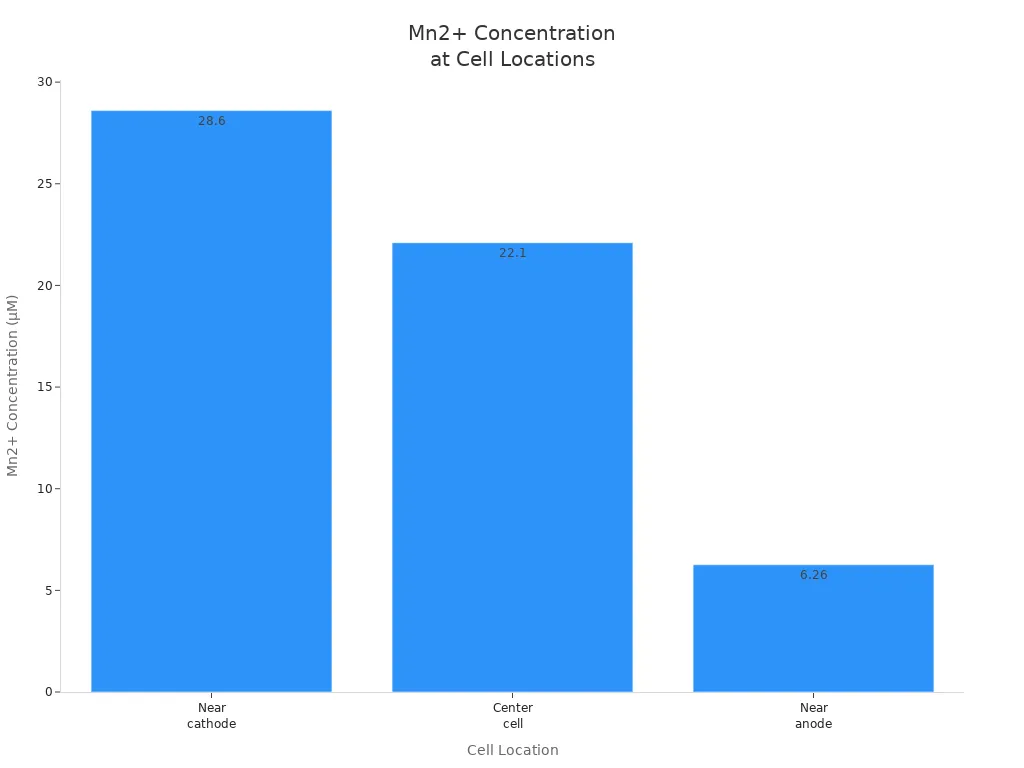
Mn2+ concentration near cathode | 28.6 μM (MRI), 8.42 × 10⁻¹⁰ mol in 2.94 × 10⁻⁵ L volume |
Mn2+ concentration center cell | 22.1 μM (MRI), 6.51 × 10⁻¹⁰ mol in 2.94 × 10⁻⁵ L volume |
Mn2+ concentration near anode | 6.26 μM (MRI), 8.45 × 10⁻¹⁰ mol in 13.5 × 10⁻⁵ L volume |
Total Mn2+ estimated (MRI) | 2.34 × 10⁻⁹ mol |
Total Mn2+ estimated (ICP-AES) | 20.6 × 10⁻⁹ mol (after 3 cycles) |
Effect of alternative electrolyte | MCP solvent with LiTFSI salt lowers Mn2+ loss compared to regular LiPF6 EC:DMC |
There are some problems when using manganese monoxide in lithium batteries. Manganese atoms can move and oxygen can leave. This can change the battery’s structure and lower how much energy it holds. The electrode can get thick layers that slow down lithium ions. Bad reactions at high voltage can block lithium-ion paths. Metal ions can move to the anode and make the battery work worse. These issues make it hard to get the best from manganese. But using coatings and things that help conduct electricity can fix some problems.
Cathode Stabilization
Cathode stabilization is a big benefit of manganese monoxide in batteries. Manganese dioxide and manganese monoxide help stop batteries from getting too hot. This makes batteries safer. Using manganese dioxide makes the cathode stronger. The battery can be charged more times and still work well.
Manganese dioxide also helps stop bad reactions with the electrolyte. Coatings on the surface act like shields. They stop unwanted reactions and keep the electrode safe. This keeps ions moving and the battery working for a long time. Manganese monoxide does not conduct electricity very well by itself. But mixing it with carbon or other materials helps. This gives better battery life and more charges. Manganese dioxide and manganese monoxide are important for new battery designs.
Benefits of Manganese Batteries
Energy Density
Manganese batteries can hold a lot of energy. This makes them a good choice for many things. Manganese dioxide in the cathode lets batteries store more energy in less space. This is important for devices that need to last longer before charging again. In nickel-manganese-cobalt batteries, manganese dioxide helps even more. These batteries can reach up to 220 Wh/kg. That is higher than many older batteries. The chart below shows how much energy different batteries can hold:

NMC batteries use manganese dioxide and have good power and safety. This makes them great for electric cars and small electronics.
Cycle Life
Manganese batteries last a long time. They can be charged and used many times before they stop working well. This means your devices or cars will last longer. You will not need to change batteries as often. Manganese helps keep the battery safe and stable. It lowers the chance of getting too hot. Big car companies use batteries with a lot of manganese now. This makes batteries safer and more reliable. You get batteries that work well even after lots of use.
Cost and Sustainability
Manganese batteries cost less because manganese is cheaper than cobalt. Manganese dioxide is easy to find and there is plenty of it. This makes batteries cheaper for everyone. Manganese batteries are also better for the environment. Mining manganese uses less energy and makes less pollution than some other metals. Recycling batteries with manganese dioxide helps save materials and cuts down on waste. But mining can still hurt water and cause pollution if not done right. Scientists are working to make manganese batteries even better for the planet.
Tip: Picking batteries with manganese dioxide helps the earth and supports fair mining.
Innovations in Battery Chemistries
Lithium-Ion and Li-S Batteries
Big changes happen in lithium batteries because of manganese dioxide. In lithium-ion batteries, manganese dioxide helps batteries hold more energy. It also makes batteries safer. You get steady voltage and batteries last longer. Lithium-sulfur batteries use manganese dioxide to slow bad reactions. This helps batteries work well for more cycles. Manganese dioxide lets batteries charge quickly and keeps them from getting too hot. If you pick lithium batteries with manganese dioxide, your devices and cars get strong and steady power.
Sodium-Ion and Magnesium Batteries
Sodium-ion batteries are more popular in 2025. Manganese monoxide and manganese dioxide are important in these batteries. Scientists use manganese oxides to make batteries last longer and work better. You get batteries that can be used many times. The table below shows how manganese helps sodium-ion and magnesium batteries:
Battery Type | Advancement Focus | Specific Evidence |
|---|---|---|
Sodium-ion (Na-ion) | Cathode materials with manganese monoxide | Using manganese oxides makes batteries more stable and strong. Na0.32[Fe0.13Mn0.40]O2 has high capacity and lasts a long time. New ways to make these batteries fix problems like Fe moving around. |
Magnesium (Mg) | Electrolyte development for Mg-ion conduction | Solid electrolytes help ions move and make batteries stable. Special polymer electrolytes let magnesium move back and forth. Mixing different electrolytes makes batteries work better. |
Magnesium batteries are getting better with new electrolytes. Manganese dioxide is not used much in these batteries. Sodium-ion batteries with manganese dioxide work well and are a good deal.
Manganese Oxide Composites
Manganese oxide composites change how batteries work. Manganese dioxide mixed with graphene helps batteries hold more energy and last longer. The chart below shows how much energy different manganese oxide composites can hold:
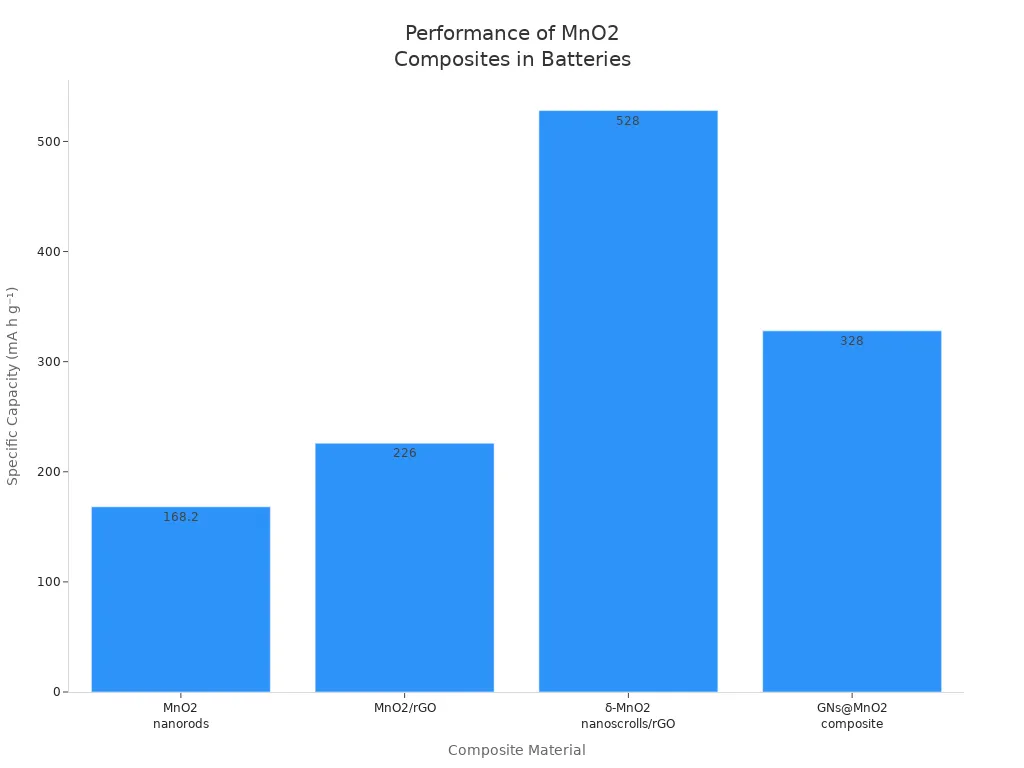
Composites like MnO2 with graphene and δ-MnO2 nanoscrolls with rGO hold more energy and last longer. The table shows what makes these composites special:
Performance Characteristic | Value / Description |
|---|---|
Specific Capacitance | |
Rate Capability | 64% capacitance stays after charging fast |
Cycling Stability | About 88% capacitance stays after 10,000 cycles |
Energy Density | 25 Wh kg⁻¹ |
Power Density | 16 kW kg⁻¹ |
Morphology | Porous shapes help ions move and reach the electrolyte |
Conductivity Enhancement | Adding nitrogen-doped carbon makes batteries work better |
Material Advantages | Many oxidation states, high capacity, low cost, good for the planet, easy to find |
Tip: Manganese dioxide composites help batteries last longer and charge faster. These materials also make batteries bend and help the environment.
Industry Trends and Outlook
Adoption by Manufacturers
Many big battery companies use manganese dioxide and manganese monoxide quickly in 2025. Companies like Panasonic, Hitachi Maxell, and Duracell are strong in the market. Smaller companies in Asia also start using these materials. This happens because people want tiny batteries for wearables and medical devices. Manufacturers work on:
Making batteries smaller for electronics and medical implants
Building batteries that last long for factories
Making batteries hold more energy and stay safe
Using recycled materials to help the planet
Asia-Pacific, especially China, makes lots of batteries because it costs less and has good supply chains. North America and Europe grow slowly, mostly for medical and factory needs. There are more deals, partnerships, and money spent on research to make batteries better and greener. Coin lithium manganese dioxide batteries are popular because they are small and cheap.
Market and Regulatory Trends
The market for manganese dioxide and manganese monoxide batteries keeps growing. The table below shows how the market changes:
Attribute | Details |
|---|---|
Market Size (2024) | |
Market Size (2033) | USD 0.32 billion |
CAGR (2025-2033) | 4.9% |
Regional Growth Leader | Asia Pacific |
Key Growth Drivers | Battery demand, EV adoption, renewable energy storage |
More people need energy, buy electric cars, and want clean energy storage. Companies like Panasonic, LG, Samsung, BYD, and CATL make more kinds of batteries. Rules help shape the battery industry. OSHA makes strict safety rules for manganese compounds. So, companies buy better safety gear. EPA and other groups want cleaner factories, more recycling, and green materials. The U.S. Inflation Reduction Act and European Green Deal help battery tech grow. This makes manganese dioxide batteries more popular.
Future Research
Scientists will keep working to make manganese dioxide and manganese monoxide batteries better. They try to:
Help batteries last longer by fixing problems
Charge batteries faster for quick use
Make batteries safer with new designs
Make recycling easier and safer for nature
Researchers also study new battery types, like manganese-hydrogen rechargeable batteries. These could help store lots of energy for the power grid. They look for cheaper catalysts to save money and meet energy goals. More work will be done to improve manganese oxide shapes, use doping, and learn why batteries stop working. This helps batteries last longer.
Manganese dioxide makes batteries better in 2025. Companies use it to make batteries safer. Batteries work well and cost less. Electric cars last longer and charge quickly. Many companies pick manganese dioxide for new batteries. Scientists will keep studying ways to use manganese dioxide. They want to make batteries even better.
Tip: Look out for new ideas with manganese dioxide that will change batteries in the future.
FAQ
What makes manganese monoxide important for batteries in 2025?
Manganese monoxide helps batteries work better. It lets electrodes move energy faster. It also keeps batteries safe from problems. Companies use it to make batteries cost less. It helps batteries store more energy.
How does manganese monoxide improve battery safety?
Manganese monoxide makes the cathode stronger. This stops batteries from getting too hot. It lowers the chance of short circuits. Your devices can work well and stay safe.
Can you recycle batteries with manganese monoxide?
You can recycle batteries with manganese monoxide. Manganese is simple to get back and use again. Recycling saves resources and helps the planet.
Are manganese batteries more affordable than cobalt batteries?
Manganese batteries cost less than cobalt batteries. Manganese is cheaper to buy. Companies use it to make batteries that are not expensive.
Which industries use manganese monoxide batteries most?
Electric cars use manganese monoxide batteries a lot. They are also in phones and other electronics. Energy storage systems use them too. Companies like these batteries because they last long and work well.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





