Manganese monoxide in steelmaking plays a crucial role in modern steel production. Metallurgical studies reveal several key functions of manganese monoxide in steelmaking:
It serves as a primary intermediate oxide at high temperatures.
It aids the reduction process, which regulates oxygen levels in steel.
By adding manganese, it enhances the strength and hardness of steel.
It acts as an internal oxygen getter, helping to remove oxygen from the steel.
Despite the benefits of manganese monoxide in steelmaking, environmental challenges remain. Although energy efficiency has improved, emissions and waste continue to pose significant issues. Therefore, effective management is more important than ever in steel production involving manganese monoxide.
Manganese monoxide takes out oxygen and sulfur from steel. This makes steel stronger and easier to shape. – When manganese is added, steel gets stronger and tougher. It also does not rust as easily because its inside changes. – New ways to make steel use less energy. They also make less pollution. This helps the planet and makes steel faster. – Steelmakers watch manganese levels closely with special alloys. This helps them make good steel and waste less. – In the future, steelmaking will use smart technology and recycling. This will make steel better and help the environment.
Manganese Monoxide in Steelmaking
Key Roles
Manganese monoxide in steelmaking has many important jobs. It helps make steel better and faster. When smelting happens, manganese monoxide forms from other manganese oxides. This compound mixes with slag and other oxides. It helps control what is in the slag. Manganese monoxide changes how acidic or basic the slag is. This affects how much manganese is saved and how good the steel is. If manganese monoxide meets acidic things like silica, it can stop reduction. Steelmakers fix this by using ores with more basic stuff or by adding roasted limestone. This makes more slag. The slag can take in manganese, which means less metal is saved.
In factories, the amount of manganese in steel must be just right. Manganese monoxide helps control this by joining reactions that remove bad elements. For example, manganese monoxide joins with sulfur in hot steel. This makes manganese sulfides. This is good because it makes steel bend better and stops sulfur from causing problems. Because manganese monoxide works with slag and impurities, it is very important in making steel today.
Note: Getting the most manganese back depends on keeping slag chemistry balanced and watching manganese levels during the process.
Why MnO is Used
Steelmakers pick manganese monoxide in steelmaking for its special properties. As a deoxidizer, manganese monoxide reacts with oxygen in hot steel. It makes manganese oxides that mix with slag. This takes out oxygen and stops gas bubbles and other problems. The steel is better and has fewer mistakes. The main reaction looks like this:
Mn + O₂ → MnO₂
This step cleans the steel and makes it stronger.
Manganese monoxide also helps remove sulfur. Manganese in steel reacts with sulfur to make manganese sulfides. This stops sulfur from making steel break easily. Adding manganese, usually as ferromanganese or ferro-manganese alloys, spreads it out and helps with deoxidation and alloying.
The table below shows the main jobs of manganese monoxide in steelmaking:
Role in Steelmaking | Explanation |
|---|---|
Deoxidation | Manganese monoxide takes away oxygen by making oxides that leave with slag. This makes steel cleaner and with fewer problems. |
Desulfurization | Manganese joins with sulfur to make manganese sulfides. This helps steel bend and be tough. |
Alloying | Manganese keeps steel strong, makes grains smaller, and helps steel be harder and tougher. |
Addition Method | Steelmakers add manganese as ferromanganese or ferro-manganese alloys when melting. |
Mechanical Effects | Manganese makes steel stronger, tougher, and better at resisting wear by changing its structure. |
Manganese monoxide in steelmaking saves money and works better than other deoxidizers. Manganese alloys like silicomanganese mean steelmakers do not need to use more expensive deoxidizers. This lowers costs. Using manganese-based deoxidizers also helps the environment. It uses less energy and makes less waste. Making manganese powders into briquettes helps use more of the material and saves money.
Manganese alloys like ferromanganese and ferro-manganese help make steel grains smaller and stronger.
Silicon manganese is a good deoxidizer. It makes both MnO and SiO₂, which helps clean the steel.
Using manganese-based deoxidizers helps the environment by making less waste and using less energy.
Steelmakers use manganese monoxide in steelmaking because it works well, saves money, and is better for the environment. By watching manganese levels and using ferromanganese and ferro-manganese wisely, steelmakers make sure their steel is high quality and not too expensive.
Chemical Properties
Structure and Reactivity
Manganese monoxide (MnO) has a crystal structure called “rock salt cubic.” In this structure, manganese and oxygen atoms repeat in a pattern. This makes MnO stable when it gets very hot. The atoms are packed close together. This helps MnO stay strong during steelmaking.
MnO can react with other things in the furnace. It mixes with iron oxide (FeO) to make a solid solution. This means MnO and FeO blend together and do not form layers. The mix stays stable even if the heat goes up. This helps steelmakers control slag and steel chemistry.
Note: MnO can mix with FeO to help control the steelmaking process.
High-Temperature Behavior
When it is very hot in steelmaking furnaces, manganese monoxide acts in special ways:
MnO forms as the main oxide when Fe–Mn alloys get oxidized between 950°C and 1150°C.
MnO and FeO make a solid solution with a rock salt cubic structure at the place where metal and oxide meet.
Oxidation starts with a straight growth stage. Oxygen moves through the gas to the alloy surface. The rate gets faster if there is more oxygen or faster gas flow.
Later, or with more oxygen, the growth becomes parabolic. Now, the speed depends on how fast atoms move through the oxide layer.
The oxide layer made at these temperatures is mostly wüstite (Fe(Mn)O). When the steel cools, this layer changes into magnetite.
The thickness and shape of the oxide layer change with heat and oxygen. This shows MnO can change a lot during steelmaking.
Steelmakers use these facts to control oxidation and make better steel. MnO’s behavior at high heat helps stop bad reactions and keeps steel strong and clean.
Manganese in Steel Production
Deoxidation
Manganese helps take out oxygen from melted steel. Steelmakers add manganese using ferromanganese or ferro-manganese alloys. These help control how much manganese is in the steel. This makes the steel better at the end. Most manganese made is used in making iron and steel. This shows how important manganese is for steel.
Manganese is a strong deoxidizer. It reacts with oxygen to make oxides that do not break down.
Using composite deoxidizers, like titanium and zirconium together, helps manganese make more manganese sulfide (MnS).
Composite deoxidizers can make over 80% of MnS start on other things, but single deoxidizers do not work as well.
Titanium oxide gathers where the steel starts to harden. This helps MnS form in the right spots.
The right amount of manganese and sulfur controls how many MnS pieces form, what they look like, and when they show up.
Steelmakers use ferromanganese and ferro-manganese to get the manganese level just right. This helps make steel with fewer problems and better strength. Careful deoxidation makes steel cleaner. Clean steel is needed for many uses in factories and building things.
Tip: Changing manganese and sulfur levels, and how fast the steel cools, helps control when and how MnS forms. This is very important for making good steel.
Desulfurization
Manganese monoxide helps take out sulfur from steel. Sulfur can make steel weak and easy to break. Adding manganese, usually as ferromanganese or ferro-manganese, raises the manganese level. This helps make manganese sulfide. Manganese sulfide leaves the steel and goes into the slag. This makes the steel stronger and easier to bend.
Studies show manganese oxides, like manganese monoxide, are good at grabbing sulfur. When manganese sulfide changes to manganese monoxide and sulfur dioxide, the right amount of oxygen is needed. This keeps the reaction safe and protects the machines. Manganese monoxide also helps speed up sulfur removal. Using more than one step to remove sulfur can take out 40% to 90% of the sulfur if done right. Adding manganese oxides, like MnO2, can help take out even more sulfur, sometimes over 90%.
Steelmakers use ferromanganese and ferro-manganese to keep the manganese level right for taking out sulfur. This helps make sure the steel is strong and can bend without breaking.
Alloying Effects
Manganese is an important alloying element in steel. Changing how much manganese is in steel changes how the steel looks inside and how it acts. Steels with lots of manganese are strong and can bend well. These good things happen because of special ways the steel changes shape, like twinning-induced plasticity (TWIP). TWIP steels make tiny twins inside the grains. These twins stop the steel from breaking and help it bend.
Adding manganese, often with ferromanganese or ferro-manganese, makes new microstructures. For example, in ODS steels with 25% manganese, TiMn2O4 nanoparticles form and stick well to the austenite. This change turns the steel from ferrite to austenite and εhcp-martensite. When the steel is stretched, many twins form, making it even stronger and easier to bend.
Alloying Effect | Microstructural Changes | Mechanical Property Impact |
|---|---|---|
TiMn2O4 nanoparticles form and connect to austenite; steel changes from ferrite to austenite and εhcp-martensite; many twins form when pulled | Steel bends more at room temperature; it is tougher in cold than steel without manganese |
Ferromanganese and ferro-manganese help steelmakers control manganese levels very well. This makes steel strong, bendy, and tough, even when it is cold. Because of this, manganese is very important in making steel today.
Production Methods

Traditional Processes
Steelmakers have made manganese monoxide for many years. They use carbothermic reduction in special furnaces. Carbon is the main thing that takes away oxygen. Manganese ores are heated with coke. This removes oxygen and makes manganese monoxide. This method uses a lot of energy, about 2000 to 3000 kWh for each ton. It also makes a lot of carbon dioxide, about 1.0 to 1.4 tons for every ton of metal. Some manganese is lost to slag, so workers must watch the process closely. Factories add ferromanganese or ferro-manganese to change how much manganese is in the steel. Low-carbon ferromanganese helps make steel that is very pure.
Sustainable Approaches
New ways try to use less energy and make less pollution. The HAlMan process uses hydrogen to change manganese oxides into manganese monoxide. This makes water vapor instead of carbon dioxide. Then, aluminum changes the MnO into manganese metal. This process uses about half as much energy as old ways. It almost stops CO2 from being made, especially if it uses green energy and recycled aluminum. Methane-based reduction also uses less energy and makes less pollution, but it still uses some carbon. The table below shows how these ways compare:
Production Process | Energy Consumption (kWh/ton metal) | CO2 Emissions (t CO2/ton metal) | Key Features |
|---|---|---|---|
Traditional SAF (carbothermic) | 2000–3000 | 1.0–1.4 | Uses lots of energy; makes lots of CO2; more manganese lost |
Methane-based reduction | Less than SAF | Less than SAF | Uses less energy and makes less pollution; still uses some carbon |
HAlMan process | 1000–1500 | Almost zero | Uses hydrogen and aluminum; saves energy; less manganese lost |
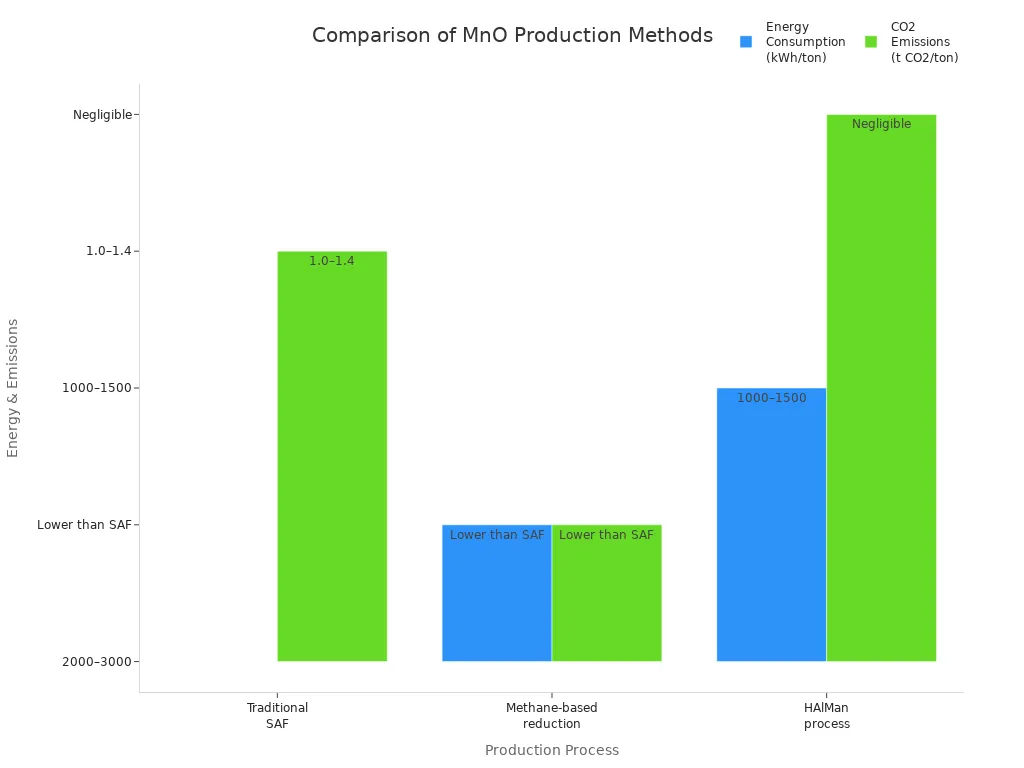
The HAlMan process is special because it uses less energy and makes almost no pollution.
Incorporation into Alloys
Steelmakers put manganese into steel using ferromanganese, ferro-manganese, or low-carbon ferromanganese. These alloys help control how much manganese is in the steel and make the steel better. Low-carbon ferromanganese is good for making very pure steel. Workers mix these alloys when melting the steel to get the right mix of strength and bendiness. Watching how much manganese is added helps stop waste and makes the process work better.
Steel Properties
Manganese monoxide changes how steel acts when stressed. When it is added, steel can stretch more before breaking. The ultimate tensile strength goes down a little. For example, in 10Ni steel, the strength drops from 582 MPa to 566 MPa. But the steel can stretch more, from 37% to 45%. This means the steel is tougher and does not snap easily at room temperature. At high heat, manganese monoxide can make steel less tough. It also shortens how long steel lasts under stress. In 12Ni steel, stretching at 650°C drops from 35% to 21%. Creep life at 750°C falls from 817 hours to 305 hours. These changes happen because manganese changes how strong parts form inside the steel.
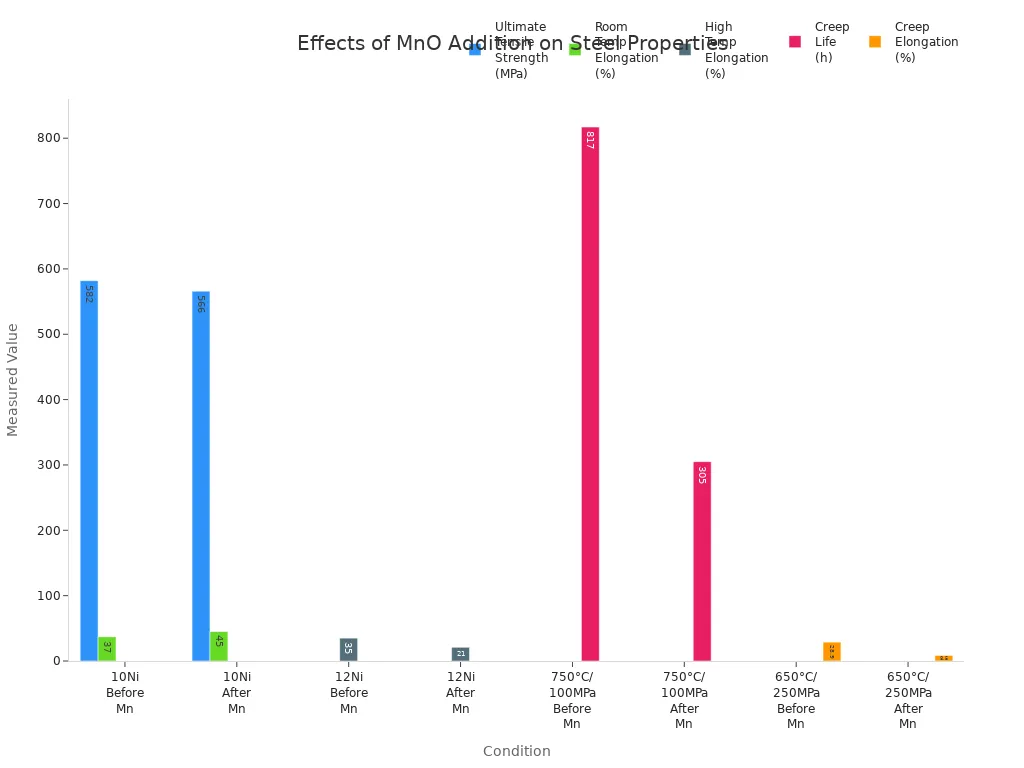
Engineers must pick the right amount of manganese to get the best mix of strength and toughness for each job.
Corrosion Resistance
Manganese monoxide helps steel resist rust. In places near the ocean, manganese makes new compounds in the rust. Sometimes, this makes the steel less safe from rust. But with chromium, manganese makes a thick rust layer. This layer protects steel from salt and water. Tests in salty water show manganese and chromium make the rust grains smaller. They also make more goethite, which sticks well and slows rust. Too much manganese can make spots that break the rust layer. This can cause small holes called pits. How well manganese monoxide stops rust depends on how much is used and what else is in the steel. Careful planning helps manganese make steel resist rust without new problems.
Microstructure
Manganese monoxide changes how steel looks inside. It affects how grains and phases form. It raises stacking fault energy, so dislocations move easier. This makes steel bend more without breaking. In some steels, manganese helps twins form inside grains. These twins stop cracks and help steel bend. In steels with 25% manganese, special nanoparticles form. They stick to the austenite phase and make steel tougher in the cold. These changes show why manganese monoxide is important for making steel strong and flexible.
Processing and Challenges
Handling manganese monoxide safely keeps workers safe and the product good. Workers follow rules to lower risks from dust and fumes. They do many things to stay safe:
Work in places with good air flow and closed spaces to keep dust down.
Wear safety gear like masks, goggles, gloves, and special clothes.
Keep manganese monoxide in cool, dry, closed containers, away from things that can cause fires.
Use fans and keep work areas clean to stop dust from spreading.
Clean up spills with special vacuums that trap dust, not brooms.
Check the air often and follow safety rules for how much manganese is safe.
Give quick help if someone breathes in, eats, or touches manganese monoxide.
Do not eat, smoke, or use tobacco where you work; wash your hands before breaks.
Throw away waste the right way, following all the rules.
Tip: Checking the air often and wearing the right masks helps keep workers safe from too much manganese.
Process Optimization
Making the process better helps use manganese monoxide in steelmaking well. Engineers use different ways to get better results:
Use special math methods to find the best way to get manganese out of ore. This means changing acid strength, heat, how much solid and liquid are mixed, how fast things are stirred, and how small the pieces are.
Use oxalic acid in hydrochloric acid to help more manganese come out of the ore.
Keep important things steady: hydrochloric acid at 1.2 mol/L, heat at 60°C, stirring at 400 rpm, pieces at 115 µm, and let it react for 120 minutes.
Make machines and mixers that keep these things the same when making more steel.
Use smart control systems and check things in real time to keep the process steady and the product good.
Learn how much energy is needed to save power when getting manganese out.
These steps help steelmakers get more manganese, spend less money, and use resources wisely. Having enough manganese makes steel better and helps it work well.
Environmental Impact
Emissions and Waste
Making steel with manganese monoxide makes pollution and waste. Factories let out carbon dioxide, sulfur oxides, and dust when they process manganese ores. These gases make the air dirty and add to climate change. Waste slag has leftover manganese and other metals. If not handled right, it can hurt the soil and water.
Steelmakers often use carbothermic reduction. This burns coke and makes a lot of carbon dioxide. For each ton of manganese alloy, old ways can make up to 1.4 tons of carbon dioxide. Waste slag builds up near steel plants. When it rains, water can wash metals from slag into rivers and lakes. This can harm plants and animals living there.
Good waste control lowers these dangers. Factories now use lined places to store waste and clean water before letting it go. They also reuse slag in building roads or making concrete.
Eco-Friendly Innovations
Many companies now try cleaner ways to make steel with manganese monoxide. Titus Steel is a leader. They make wear-resistant steels without making any carbon dioxide. They use hydrogen instead of coke to process manganese ores. This makes water vapor, not carbon dioxide. Titus Steel also recycles carbon dioxide by turning it into carbon monoxide for blast furnaces. These steps help lower pollution and make the process greener.
Another new idea is making low-carbon ferromanganese in one step using an electric arc furnace. This uses pre-reduced manganese ore and needs as little as 410 kWh for each ton. It also cuts carbon dioxide by about 1,560 kg per ton compared to old ways. This saves raw materials and makes the alloy better.
Innovation | Main Benefit | Environmental Impact |
|---|---|---|
Hydrogen reduction | Zero CO2 emissions | Cleaner air, less waste |
EAF single-step LC-FeMn | Lower energy and emissions | Less CO2, saves resources |
These green changes show steelmakers can help the planet and still make strong, good steel.
Future Trends
New Technologies
Steelmakers are always looking for better ways to make steel. Many companies now use smart sensors and artificial intelligence in their factories. These tools help workers watch the furnace closely. They can control heat and chemical changes more easily. Some factories use robots to move hot steel. This keeps workers safer and stops accidents. Scientists have made new alloys with manganese. These alloys make steel stronger and easier to bend. Cars and buildings last longer with these alloys. Researchers also test 3D printing with steel powders that have manganese. This lets them make special shapes and waste less steel.
Experts think digital tools and machines will change steelmaking. Workers can see data right away and change things to save energy.
Here is a table with some new steelmaking technologies:
Technology | Main Benefit | Impact on Steel Quality |
|---|---|---|
AI Process Control | More accurate results | Fewer mistakes |
Robotics | Safer jobs | Products stay the same |
3D Printing | Custom shapes | Less waste |
Sustainable Steelmaking
Steelmakers now want to make steel in ways that help the earth. Many factories use hydrogen instead of coal for manganese ores. This makes less carbon dioxide and cleaner steel. Some companies melt old steel and manganese alloys to use again. Factories use solar and wind power to run furnaces. These choices lower pollution and save energy.
Steelmakers also find ways to use slag and other waste. They turn slag into things for roads and buildings. By recycling and using clean energy, steelmaking hurts nature less.
Making steel in a green way helps companies follow new rules. It also keeps the earth safe for people in the future.
Manganese monoxide is very important in making steel and alloys.
It helps make steel that is strong, lasts long, and does not rust easily.
Manganese alloys make steel tougher and help create stronger steels and other alloys.
Companies are now trying to use cleaner ways that save energy and make less pollution and waste.
As more steel is needed and new batteries are made, manganese will keep being important for new ideas and a greener industry.
FAQ
What is manganese monoxide used for in steelmaking?
Manganese monoxide helps take out oxygen and sulfur. It acts as a deoxidizer and desulfurizer. This makes the steel stronger and easier to bend. The steel is better and lasts longer.
How does manganese monoxide improve steel properties?
Manganese monoxide changes how steel looks inside. It makes steel tougher and stronger. It helps steel fight rust and wear. Engineers use it to make steel safer and last longer.
Is manganese monoxide safe to handle?
Workers must follow safety rules at work. They wear masks, gloves, and special clothes. Good air flow is needed in the factory. Breathing manganese monoxide dust can hurt your lungs. Factories teach workers how to stay safe.
Can steelmakers recycle manganese monoxide waste?
Yes, steelmakers can recycle waste with manganese monoxide. They use special ways to get manganese back. This helps save materials and lowers waste.
What are the environmental benefits of new manganese monoxide production methods?
New ways use less energy and make less pollution. Hydrogen reduction lowers carbon dioxide emissions. These methods help keep the earth clean. They support green steelmaking.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





