Many people get mixed up when they look at manganese dioxide vs oxide. Manganese dioxide is a certain compound with the formula MnO2. Manganese oxide can be any oxide form of manganese, like MnO or Mn3O4. This difference is very important. It matters when someone needs the right material for a job or process. For example, in water treatment and fuel cells, the type of manganese oxide changes how well things work. In medicine, manganese dioxide nanosheets help make special sensors and drug delivery systems. This is because of their special structure and redox abilities. Purity and how each manganese oxide is made also change how they work in science and industry.
Key Takeaways
Manganese dioxide (MnO2) is one compound. It has manganese in the +4 oxidation state. Manganese oxides are a group of compounds. They have different oxidation states and properties.
Manganese dioxide is black and stable. It is a strong oxidizer. This makes it good for batteries, water treatment, and catalysts. Other oxides like MnO and Mn3O4 look different. They have other colors and uses. Some are used in ceramics and pigments.
There are different types of manganese dioxide. Each type has a special structure. This changes how they store energy and work as catalysts. Picking the right type is important for your project.
Purity and how manganese dioxide is made matter a lot. This is very true for batteries. High purity electrolytic manganese dioxide works best in them.
When you buy manganese compounds, check some things. Look at the oxidation state and purity. Make sure the supplier is reliable. Check for certifications. This helps you get the right material for your needs.
Manganese Dioxide Vs Oxide
Main Differences
When people talk about manganese dioxide vs oxide, they mean one compound and a group of chemicals. Manganese dioxide is just one compound. Its formula is MnO2. It has manganese in the +4 oxidation state. Other manganese oxides, like manganese(ii) oxide (MnO) or Mn3O4, have different formulas. They also have different oxidation states. These differences change how each one looks, reacts, and what it can do.
Manganese dioxide is special because it is a strong oxidizer. It has a stable structure. It looks black or dark brown. Its crystal structure is made of MnO6 octahedra. This gives it high surface energy. It is great for batteries and as a catalyst. Other manganese oxides, like manganese(ii) oxide, are green or pink. They have simpler structures. They are less stable. They have lower oxidation states. This means they react differently.
The oxidation state is very important for manganese. Manganese dioxide has a +4 oxidation state. It is a strong oxidizer. It helps in reactions that need oxygen transfer. Manganese(ii) oxide has a +2 oxidation state. It is not as strong. It is more basic and less reactive. Mn3O4 has both +2 and +3 oxidation states. It is in the middle. These changes affect how each compound works. For example, manganese dioxide is best for battery cathodes and water treatment. Manganese(ii) oxide is used in ceramics and to make other chemicals.
Crystal structure matters too. Manganese dioxide comes in many forms. These include α-, β-, γ-, δ-, and ε-MnO2. Each form has a different shape and surface area. δ-MnO2 has a layered structure. Water and alkali ions sit between the layers. This helps move ions and store energy. β-MnO2 has tunnel structures. These limit ion movement but make it very stable. Manganese(ii) oxide has a rock-salt structure. It is simpler and not good for energy storage.
You can tell these compounds apart by their color. Manganese dioxide is black or dark brown. Manganese(ii) oxide is green or pink. Mn3O4 is brownish-black. These colors come from their structures and oxidation states.
How these compounds react is also different. Manganese dioxide is stable. It does not react much with air or water. It can break down hydrogen peroxide and make oxygen. Manganese(ii) oxide is less stable. It can change when it touches air or water. Mn3O4 is even less stable. It forms when manganese metal reacts with air.
The differences between manganese dioxide and oxide matter in real life. Manganese dioxide is used in batteries, pigments, and water treatment. Other manganese oxides, like manganese(ii) oxide, are used in ceramics, fertilizers, and as catalysts. The oxidation state, structure, and stability decide where each one is used.
Tip: Always check the oxidation state and structure before picking a manganese compound. These details change how well it works for your project.
Comparison Table
Here is a quick look at manganese dioxide vs oxide. It includes manganese(ii) oxide and Mn3O4:
Feature | Manganese Dioxide (MnO2) | Manganese(II) Oxide (MnO) | Manganese(II,III) Oxide (Mn3O4) |
|---|---|---|---|
Chemical Formula | MnO2 | MnO | Mn3O4 |
Manganese Oxidation State | +4 | +2 | +2, +3 |
Crystal Structure | Rock-salt (MnO6 octahedra) | Spinel (octahedral & tetrahedral) | |
Physical Appearance | Black or dark brown powder | Green or pink powder | Brownish-black powder |
Chemical Behavior | Strong oxidizer, stable | Basic, less reactive, less stable | Moderate reactivity, less stable |
Surface Energy | High, good for catalysis and batteries | Lower | Lower than MnO2 |
Main Applications | Batteries, pigments, water treatment, catalysts | Catalysts, pigments, ferrites |
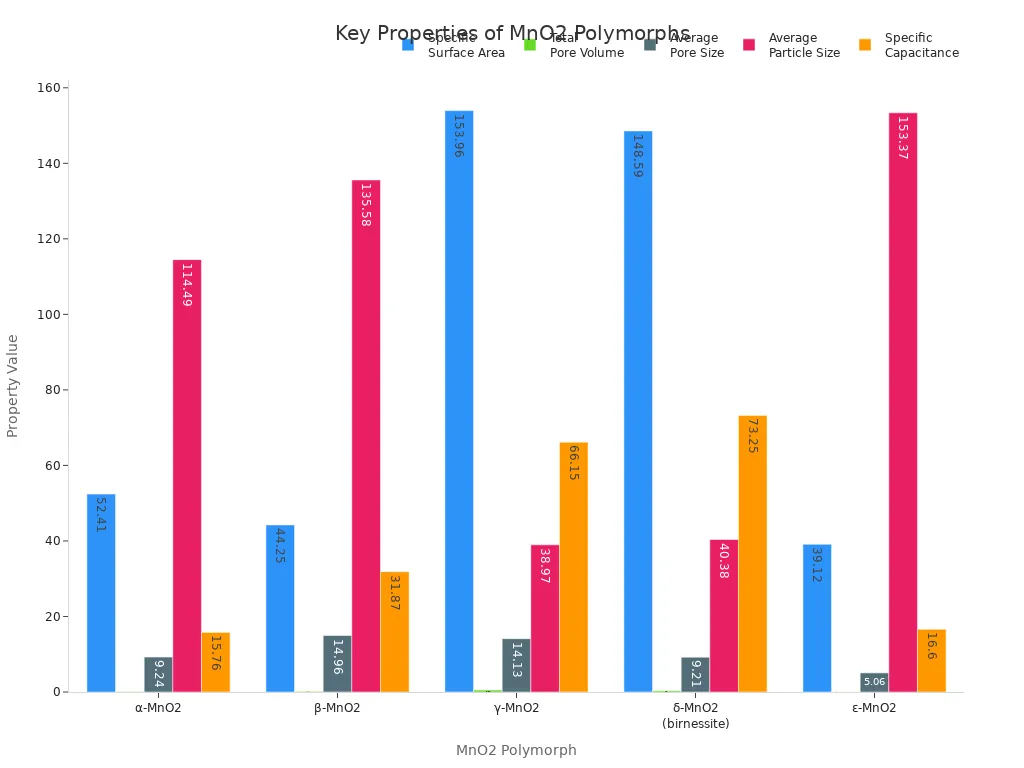
Manganese dioxide comes in many forms. Each has its own features. The chart below shows how different MnO2 forms compare in surface area, pore size, and capacitance. These things matter for energy storage and catalysis.
Each form of manganese dioxide has a special structure. For example, δ-MnO2 has a layered structure. Water and ions sit between the layers. This helps ions move easily. It is great for supercapacitors. β-MnO2 has a tunnel structure. It has the most oxygen vacancies. This makes it good for some reactions. γ-MnO2 has a large surface area. It works well in batteries. These differences show why manganese dioxide vs oxide is important for anyone working with manganese compounds.
Definitions
Manganese Dioxide
Manganese dioxide is one special compound. Its formula is MnO2. People see it as a black or dark brown powder. It has manganese in the +4 oxidation state. Scientists and factories use manganese dioxide in many ways. It is a strong oxidizer. It helps in batteries, water cleaning, and as a pigment.
There are different forms of manganese dioxide. These are called polymorphs. Each form has its own crystal structure. For example, α-MnO2 has tunnels. δ-MnO2 has layers. These shapes change how it stores energy and reacts. Electrolytic manganese dioxide and chemical manganese dioxide are two main types. Electrolytic manganese dioxide is made by an electrolytic process. This makes it very pure and gives it a special structure. It is great for batteries. Chemical manganese dioxide is made by chemical reactions. It is often used in water cleaning.
Tip: Always check if you need electrolytic or chemical manganese dioxide for your project. The right type can help your project work better.
Manganese Oxides
Manganese oxides are a group of compounds. Each one has a different amount of oxygen. They also have different manganese oxidation states. These oxides can be green, brown, or black. Scientists sort them by oxidation state and crystal structure. Some common types are MnO, Mn2O3, and Mn3O4. Each type has its own uses and features.
Here is a table that shows how scientists sort manganese oxides:
Manganese Oxide Type | Oxidation State | Crystal Structure / Polymorphs | Structural Features | Classification Criteria |
|---|---|---|---|---|
Mn(II) | Rock salt structure | Mn(II) cations octahedrally coordinated by O2− | Based on oxidation state and rock salt structure | |
Mn2O3 | Mn(III) | α-Mn2O3 with cubic bixbyite structure | Contains Mn(III) cations | Oxidation state and bixbyite crystal structure |
MnO2 | Mn(IV) | Six or more polymorphs (tunnel, layered) | Mn(IV) cations in MnO6 octahedra; different chain lengths and vacancies | Polymorph-specific features and Raman spectroscopy |
Birnessite | Mixed Mn(III)/Mn(IV) | Layered MnO6 octahedra with vacancies | Hydrated layers, vacancies, water or cations between layers | Raman vibrational modes and structural vacancies |
Hollandite | Mixed Mn(III)/Mn(IV) | Tunnel structure | Tunnels hold large cations or water | Tunnel structure and Raman spectral features |
Manganese oxides are important in science and industry. Electrolytic manganese dioxide is used in batteries. Other oxides, like MnO and Mn3O4, are used in ceramics, pigments, and as catalysts. Scientists sort these oxides by oxidation state, crystal structure, and how they vibrate under special light called Raman spectroscopy.
Note: Knowing the exact manganese oxide type helps people choose the best material. Each oxide has its own best use and strengths.
Sources and Preparation
Natural Occurrence
Manganese dioxide and other manganese oxides are found in many places in nature. The most common natural form of manganese dioxide is called pyrolusite. People find pyrolusite in big deposits all over the world. This mineral forms when rocks with manganese change over time. Rain and air help turn these rocks into manganese oxide minerals. Pyrolusite is the main ore used to get manganese for making things.
In the ocean, manganese makes nodules and crusts on the sea floor. These nodules cover large areas, especially in the Pacific Ocean. They have manganese dioxide and other oxides like birnessite and todorokite. These nodules grow very slowly over thousands of years. Water brings manganese from rivers, volcanoes, and hot vents to the ocean. The manganese settles and forms these nodules. Manganese oxides also show up in soil and dirt. They can look like tiny grains, thin layers, or even tree-like marks on rocks.
Did you know? Manganese nodules on the ocean floor can be as big as potatoes and have valuable metals for future mining.
Industrial Synthesis
Factories use different ways to make manganese dioxide and other manganese oxides. Each way gives different shapes and purity. Here are some common ways:
Hydrothermal Method: Workers use high heat and pressure in water to make pure manganese dioxide. This way controls size and shape well, but it needs special tools and takes a long time.
Sol–Gel Approach: This starts with metal salts that turn into a gel. After heating, the gel becomes manganese oxide. People use this for special materials, but it is not used much for big factories.
Chemical Coprecipitation: This easy and cheap way mixes chemicals to make manganese oxide particles. It saves energy but sometimes gives lower purity.
Chemical Reduction Method: Factories use reducing agents to make manganese dioxide at low heat. This way is simple and cheap, but it may use harmful chemicals.
Green Synthesis Methods: Some companies use plant extracts or microbes to make manganese oxide in a safe way for nature. These ways are better for the earth, but they may not make as much material.
Each way helps different industries. Battery makers need very pure manganese dioxide, so they pick the best methods. Other industries, like ceramics or pigments, may use easier ways to make what they need.
Physical and Chemical Properties
Color and Appearance
Manganese dioxide and other manganese oxides have different colors and shapes. Manganese dioxide is usually black or dark brown. In labs, scientists see it can look like flowers or small round balls. Some types, like Ag-doped manganese dioxide, grow in thin, flower-like shapes. Other oxides, such as Mn3O4, also make flower shapes or round particles. These shapes can be close in size, but their surfaces and how stable they are can change. For example, Mn3O4 nanoparticles have smooth surfaces but do not stay stable in water as well as some changed manganese oxides. The table below shows how these oxides are different:
Manganese Oxide Type | Appearance / Morphology | Size / Shape Details | Phase Composition | Surface Features |
|---|---|---|---|---|
MnO2 (Ag-doped) | Flower-like nanostructures | Nanoflowers ~771 nm | Mixed cubic Mn2O3, orthorhombic Mn3O4 | Fibrous surface |
Mn3O4 / Mn2O3 | Nanoflowers | ~35 nm for Mn3O4 | Cubic Mn2O3, orthorhombic Mn3O4 | Flower-like morphology |
Mn3O4 (nanoparticles) | Round-shaped nanoparticles | Similar diameter | Hausmannite phase, some impurities | Smooth, less stable in water |
Modified Mn oxide (GNA35) | Round-shaped nanoparticles | Similar to Mn3O4 | Hausmannite, purer | More stable, higher surface area |
Scientists look at color and shape to choose the best manganese oxide for batteries or other things.
Solubility and Reactivity
Manganese dioxide has special features for dissolving and reacting. It does not dissolve in water, so it stays solid. This helps in batteries because it needs to be stable. Even in most acids, manganese dioxide does not dissolve much. Sometimes, with certain chemicals, it can make stable, dissolved forms. This happens when reduction changes the oxidation state, often with help from chemicals like phosphate ions. These stable liquids can last a long time. Other manganese oxides, like Mn3O4, may react in other ways, but manganese dioxide is known for not dissolving and being a strong oxidizer. This is important for batteries, where manganese dioxide helps with the movement of zinc.
In batteries, manganese dioxide is the main part of the cathode. It helps electrons move during oxidation and reduction, working with zinc to store and give out energy.
Purity Grades
Manganese oxides come in different purity grades. These grades help different industries get what they need. For example, chemical manganese dioxide and electrolytic manganese dioxide are both used in batteries, but electrolytic manganese dioxide is much cleaner. This high purity is needed for batteries to work well and last longer. Other grades are chemical grade, fertilizer grade, and feed grade. These grades are used for paints, ceramics, and energy storage. All over the world, companies use these grades to make sure they get the right manganese oxide for their products.
High Purity
Metallurgical Grade
Battery Grade
Picking the right purity grade is very important. For batteries, electrolytic manganese dioxide is the best because it works well with zinc and lets batteries be used many times.

Cost and Availability
Price Factors
Many things can change how much manganese dioxide costs. When more batteries are made, prices often go up. This happens a lot with electric cars and energy storage. Factories that make ceramics, glass, and water treatment also use more manganese oxides. This makes the demand higher.
Here are some main things that affect price:
More batteries for electric cars and energy storage make prices rise.
Ceramics, glass, and water treatment need more manganese oxides.
The cost of manganese ore and sulfuric acid changes how much it costs to make.
Shipping and trading can make prices go up or down.
Rules for the environment and safety add extra costs for companies.
If raw materials get more expensive, prices can change a lot.
Demand in places like Asia Pacific has a big effect on price.
New ways to make manganese oxides can lower costs or make them better.
Big companies can change prices by making new deals or products.
Note: If raw materials cost more or new rules start, companies may have to pay more for manganese oxides.
Market Supply
How much manganese dioxide is available depends on how much is made and needed. The market for electrolytic manganese dioxide is growing quickly. In 2024, it was worth $2.47 billion. It could almost double by 2032. This is because more batteries and electric cars are being made.
The top countries that make manganese are South Africa, Gabon, and Australia. Here is a quick look at what they produce:
Country | Manganese Production (2024) | Notable Details |
|---|---|---|
South Africa | 7.4 million metric tons | Largest producer and holds biggest reserves |
Gabon | 4.6 million metric tons | Major supplier to the US |
Australia | 2.8 million metric tons | Third largest producer |
Some Western countries might not have enough because they mine less but need more. BRICS countries like China and India will likely keep strong supplies. Politics, trade rules, and recycling also change the market. Even though manganese is found in many places, it is still very important for batteries and green energy.
Tip: Companies that want a steady supply often work with top-producing countries or invest in recycling so they do not run out.
Applications
Manganese Dioxide Uses
Manganese dioxide is used in many industries because it has special features. The most common place you see it is in batteries. In battery factories, manganese dioxide is the main part of the cathode. It works with zinc to hold and give out energy. This is why dry-cell batteries, like those in flashlights and remotes, use manganese dioxide. It helps these batteries last longer and work better.
Here are some ways people use manganese dioxide:
It is the cathode in dry-cell and zinc-carbon batteries. It reacts with zinc to make electricity.
It is a strong oxidizer in chemical reactions.
It helps clean water by taking out iron and manganese. Water plants use it to make water safe.
It is a pigment in ceramics and glass. It gives color and makes things stronger.
High purity manganese dioxide, especially electrolytic type, is best for batteries and electronics. Lower purity is good for water cleaning and other uses.
Tip: Always check the purity when picking manganese dioxide for batteries. Higher purity means the battery will work better.
Other Oxides Uses
Other manganese oxides, like MnO and Mn3O4, have their own uses. Mn3O4 has special magnetic and electronic features. Scientists use it in labs and factories for many things.
Some main uses are:
It is a catalyst in chemical reactions and energy storage.
It is used in sensors, solar energy, and ion exchange.
It is used in batteries, sometimes as a cathode or in new battery types.
It is a pigment in ceramics and paints because of its color and strength.
It may help in medicine. Mn3O4 nanoparticles can fight germs and cancer. They also help carry medicine in the body.
It is used in new technology for optics, food safety, and cosmetics.
The table below shows how manganese dioxide and other oxides are used in batteries:
Material | Main Battery Role | Key Feature | Challenge |
|---|---|---|---|
Manganese dioxide (MnO2) | Cathode in dry-cell batteries | High energy density, stable | Needs high purity for best results |
Mn3O4, MnO, other oxides | Cathode or additive | Unique magnetic/electronic properties | May have lower stability or capacity |
Manganese oxides often work with zinc in batteries. Each oxide does something different. Manganese dioxide is the best for dry-cell batteries. Other oxides help in new battery designs and medical uses.

Choosing a Supplier
What to Look For
Picking the best supplier for manganese dioxide or other manganese oxides is very important. It can change how good your product is and if your project works well. Some suppliers are not as reliable or do not have pure materials. When looking for a supplier, buyers should remember some key things:
Good materials: Suppliers should use the best raw materials. This keeps products safe and strong.
Modern methods: New ways to make things and careful checks help keep quality the same.
Certifications: Find suppliers with ISO 9001, ISO 14001, or similar papers. These show they care about quality and the environment.
Customer help: Good suppliers answer questions fast and fix problems quickly.
Good history: Suppliers with many happy customers and on-time deliveries are more trustworthy.
Fair prices: Try to get a good deal but do not forget about quality and service.
Supply: Reliable suppliers can give you what you need on time.
Help after buying: Good support after you buy helps fix problems and keeps your project going.
Name and guarantees: Well-known suppliers often give warranties. This helps buyers feel safe.
Tip: Always ask for safety data sheets and certificates. These papers show the supplier follows rules and keeps products safe.
btlnewmaterial Overview
btlnewmaterial is a trusted company for manganese dioxide and other manganese oxides. They follow strict rules for storing and handling to keep products clean and pure. They keep materials in cool, dry places with good air flow. Containers are closed tight and have labels for easy finding. Workers check storage often to find problems early.
The company uses special tools like X-ray diffraction and scanning electron microscopy. These tools help check the crystal structure and purity of each batch. btlnewmaterial uses a first-in, first-out system so customers get the newest product. Their team trains often on safe handling and what to do in emergencies.
btlnewmaterial follows world shipping rules and uses strong packaging to keep products safe during shipping. They use clear safety labels and follow all safety steps. Customers can trust btlnewmaterial for on-time delivery, good support, and high-quality manganese dioxide every time.
Manganese dioxide is different from other manganese oxides. It has a +4 oxidation state and is very pure. It also has a stable black color. Other oxides have lower oxidation states and more impurities. It is important for buyers to know these facts. This helps them choose the right one for batteries, catalysts, or water treatment.
Chemical manganese dioxide costs more but is best when you need high purity.
Natural oxides cost less but might not meet strict quality rules.
Picking a trusted supplier like btlnewmaterial keeps things safe and works well. Always check what your project needs and talk to experts before you buy manganese compounds.
FAQ
What is the main difference between manganese dioxide and other manganese oxides?
Manganese dioxide has manganese in the +4 state. It looks black. Other oxides, like MnO or Mn3O4, have different states and colors. Each type is best for certain uses.
Can manganese dioxide be used in all types of batteries?
Manganese dioxide is best for dry-cell and alkaline batteries. Some new batteries use other manganese oxides or mixes. Always check what the battery needs before picking a material.
Is manganese dioxide safe to handle?
Manganese dioxide is safe if you are careful. Do not breathe in the dust or touch it with bare skin. Wearing gloves and a mask keeps you safe from harm.
How can someone tell if they need high-purity manganese dioxide?
High-purity manganese dioxide is best for batteries and electronics. If you want strong power or long life, pick high-purity. For water cleaning or color, lower purity is usually okay.
Where can buyers find reliable manganese dioxide suppliers?
Buyers can find good suppliers like btlnewmaterial online or from industry contacts. Look for companies with good reviews, clear papers, and helpful support.
Related Posts

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.