Lithium manganese dioxide serves as a critical battery material, combining lithium and manganese dioxide to form a stable and efficient power source. This lithium manganese dioxide chemistry has become essential for devices across key sectors, including consumer electronics, medical equipment, automotive, and energy storage systems. The global market for lithium manganese dioxide batteries reached approximately $1.3 billion in 2024, with strong growth expected in the coming years.
Metric | Value |
|---|---|
Market size in 2024 | |
Projected market size 2031 | US$ 2009 million |
CAGR (2025-2031) | 6.6% |
Largest regional market | Asia-Pacific (43%) |
Other major regions | North America (28%), Europe (26% |
Lithium manganese dioxide batteries offer high voltage and stable power, making them ideal for medical, industrial, and consumer devices.
These batteries have a long shelf life of up to 10 years with very low self-discharge, ensuring reliable performance over time.
They provide strong safety features, including resistance to overheating and thermal runaway, which protects devices and users.
The chemistry supports operation in extreme temperatures from -40°C to 60°C, suitable for harsh environments.
Compared to alkaline and lithium-ion batteries, lithium manganese dioxide batteries balance safety, energy density, and cost effectively.
What Is Lithium Manganese Dioxide
Chemistry and Structure
Lithium manganese dioxide serves as a widely used cathode material in primary batteries. Its chemical formula, often represented as LiMnO₂ or in lithium-manganese-rich variants as Li₁.₂Ni₀.₂Mn₀.₆O₂, highlights the combination of lithium ions with manganese dioxide in a layered oxide structure. This structure features complex phase behavior, including both solid-solution and chemically separated phases, which form during high-temperature processing. The presence of layered phases and phase boundaries directly impacts how lithium ions move within the material, affecting both energy density and cycling performance.
The crystal structure of manganese dioxide can appear in several polymorphic forms, each with unique properties relevant to battery applications. The table below summarizes the main forms and their characteristics:
Crystal Form
Structure Type
Surface Area
Oxygen Vacancies
Electrochemical Suitability
δ-MnO₂
Layered
Largest
Abundant
High capacitance, good for cathodes
γ-MnO₂
Tunnel
Large
Moderate
Good for batteries
α-MnO₂
Tunnel
Smaller
Less
Lower capacity
β-MnO₂
Tunnel
N/A
High
Limited suitability
ε-MnO₂
Disordered
Smaller
Less
Lower capacitance
The layered and tunnel structures in lithium manganese dioxide play a critical role in battery performance. Layered structures allow lithium ions to migrate efficiently, supporting high energy density and stable cycling. Tunnel and 3D frameworks, formed by MnO₆ octahedra, create pathways for ion transport and influence the battery’s rate capability and lifespan. Adjusting particle size and mixing different crystal phases can further enhance conductivity and increase reaction sites, leading to improved capacity and energy density.
Manufacturing lithium manganese dioxide cathodes involves precise control of moisture and temperature. The process includes mixing heat-treated manganese dioxide powder with conductive carbon and binders, coating onto a metal collector, and subjecting the assembly to heat treatment and humidity control. These steps ensure the preservation of the material’s electrochemical properties, which are essential for battery safety and long-term performance.
Battery Formats
Lithium manganese dioxide batteries come in a variety of formats to meet the needs of different applications. The most common types include button (coin), cylindrical, and rectangular (cuboid) cells. Each format offers unique advantages in terms of size, capacity, and application suitability.
Coin (Button) Cells: These compact batteries provide long life and stable voltage, making them ideal for low-drain devices such as real-time clocks, memory backup systems, and medical implants.
Cylindrical Cells: Available in standard sizes like AA, C, and D, these cells deliver higher capacities and are used in devices requiring more power, such as cameras and industrial sensors.
Pouch and Cuboid Cells: Thin and flexible, these formats suit wearable devices, IoT sensors, and other applications where space is limited.
Attribute
Details
Chemistry
Lithium manganese dioxide (LiMnO₂)
Voltage
3–3.3 V
Specific Energy
Shelf Life
10–20 years
Operating Temp.
-30°C to 60°C
Formats
Cylindrical, button/coin, cuboid, pouch
Typical Applications
Medical devices, sensors, cameras, IoT
These batteries operate reliably across a wide temperature range and exhibit low self-discharge rates, often less than 1% per year for coin cells. Their design flexibility allows manufacturers to tailor battery size and capacity for specific requirements, supporting a broad spectrum of modern electronic devices.
How It Works
Electrochemical Process
Lithium manganese dioxide batteries operate through a unique electrochemical mechanism. During discharge, lithium ions move from the anode and intercalate into the heat-treated manganese dioxide cathode. This process involves both two-phase and solid solution reactions. At first, the manganese dioxide structure transforms into a lithium-inserted phase, expanding its unit cell. As the battery continues to discharge, lithium ions insert more evenly, eventually forming a final product with a different crystal structure. These changes in the manganese dioxide lattice allow the battery to deliver energy efficiently. Researchers have confirmed this mechanism using advanced X-ray diffraction techniques, which reveal that the pathway of lithium insertion depends on how quickly the battery discharges. This combination of structural transformation and ion movement gives the battery its reliable performance and long shelf life.
Note: The ability of lithium ions to move smoothly into the manganese dioxide structure ensures stable energy output and supports the battery’s use in critical devices.
Voltage and Performance
Lithium manganese dioxide batteries stand out for their high voltage output. Each cell typically delivers about 3.0 volts, which is nearly double the voltage of common primary batteries such as zinc–carbon, zinc chloride, and alkaline types. This higher voltage supports devices that require stable and powerful energy sources, including medical equipment and advanced electronics.
Battery Chemistry | Typical Voltage (V) | Notes |
|---|---|---|
Lithium manganese dioxide (LiMnO2) | ~3.0 | Higher voltage than most primary batteries; used in high-drain devices and long shelf life |
Zinc–carbon | ~1.5 | Inexpensive, common primary battery |
Zinc chloride | ~1.5 | Also called “heavy duty” batteries |
Alkaline (zinc–manganese dioxide) | ~1.5 | Moderate energy density, good for various drain levels |
Lithium iron disulfide (LiFeS2) | ~1.5 | Expensive, used in premium batteries |
Lithium-ion rechargeable | ~3.7 | Rechargeable, higher voltage than LiMnO2 |
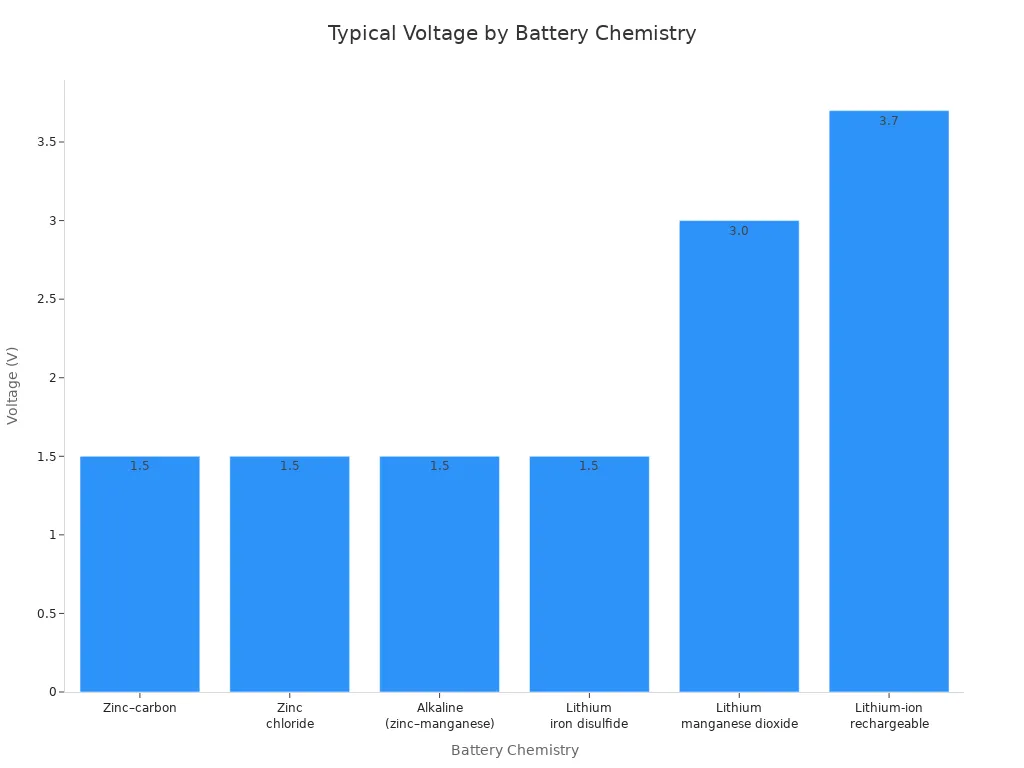
The high voltage and stable discharge curve of lithium manganese dioxide batteries make them ideal for applications where consistent performance is essential. Devices benefit from longer operating times and reduced need for frequent battery changes. This advantage, combined with the battery’s robust electrochemical process, supports its widespread use in demanding environments.
Performance and Safety
Energy Density
Lithium manganese dioxide batteries deliver impressive energy density, making them suitable for demanding applications. Their theoretical gravimetric energy density can reach up to 855 Wh/kg. In real-world use, these batteries typically provide around 300 Wh/kg by mass and 600 Wh/L by volume. Devices benefit from a high open-circuit voltage near 3 V. These batteries operate reliably across a wide temperature range, from −40 °C to +60 °C. They also maintain a low self-discharge rate, usually less than 1% per year at room temperature. The manganese dioxide cathode material remains both safe and cost-effective, which enhances overall battery performance.
High theoretical energy density (up to 855 Wh/kg)
Practical energy density: ~300 Wh/kg (mass), ~600 Wh/L (volume)
Open-circuit voltage: ~3 V
Wide operating temperature range: −40 °C to +60 °C
Low self-discharge: <1% per year
Long operational life: about 10 years
Safe, low-cost cathode material
When compared to other battery types, lithium manganese dioxide batteries offer a strong balance between energy density and safety.
Battery Type | Specific Energy (Wh/kg) | Notes |
|---|---|---|
Lithium Manganese Dioxide (Li-M) | ~280 | Moderate specific energy, safe for public use, voltage 3.0–3.3V, used in medical devices, cameras |
Lithium-ion | >200 (varies by type) | Generally higher energy density than Li-M, used in smartphones, laptops, EVs |
Alkaline | 80-100 | Lower energy density, suited for moderate energy demand devices like flashlights |
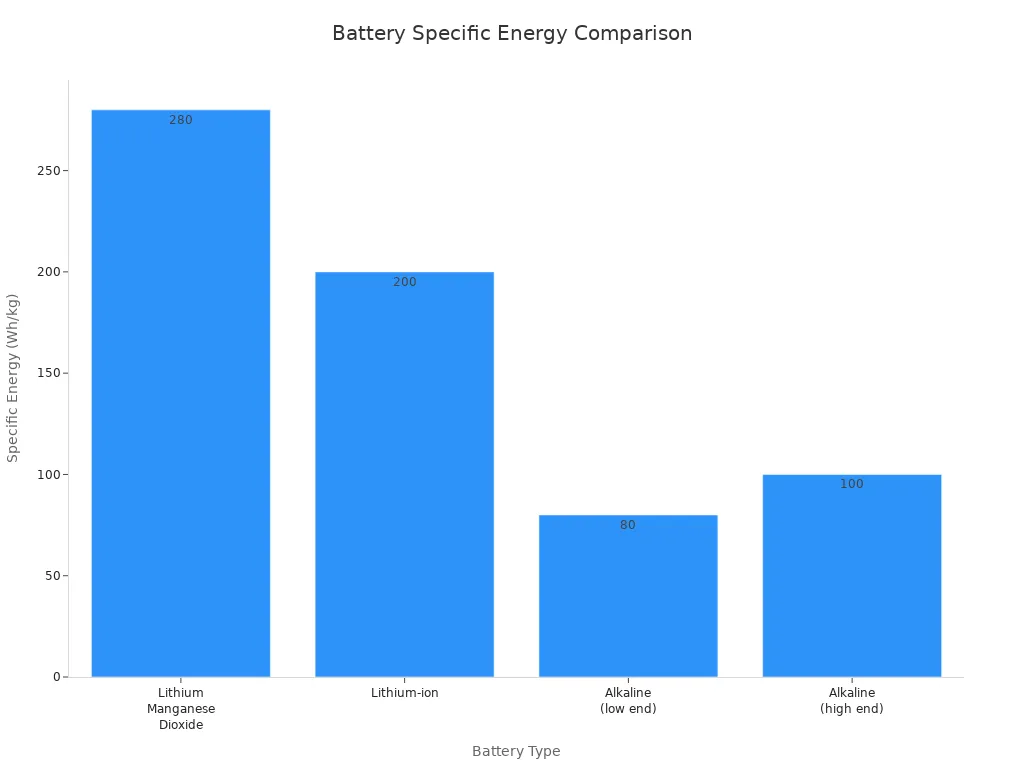
Shelf Life
Lithium manganese dioxide batteries excel in shelf life. Under standard storage conditions, primary lithium batteries, including this type, can be stored for up to 10 years with only moderate capacity loss. Manufacturers often report less than 1% self-discharge per year. Cylindrical and 9V formats typically last up to 10 years from the date of manufacture. Coin cell versions usually offer a shelf life of 5 to 7 years, with expiration dates clearly marked on packaging. This long shelf life supports their use in critical devices that require reliable, long-term power.
Battery Type | Typical Shelf Life (Standard Storage) | Additional Details |
|---|---|---|
Cylindrical & 9V Lithium MnO2 | Up to 10 years from manufacture | Less than 1% self-discharge per year |
Coin Cell Lithium MnO2 | 5 to 7 years from manufacture | Expiry date usually on packaging |
Note: The extended shelf life and low self-discharge make these batteries ideal for emergency equipment and backup systems.
Safety Features
Manufacturers design lithium manganese dioxide batteries with multiple safety features to prevent overheating and leakage. These batteries show superior thermal stability and resist thermal runaway, which reduces the risk of overheating. The amount of active material in each cell is carefully limited to prevent overloading. Built-in safety mechanisms detect abnormal conditions, such as overcharging or high temperatures, and trigger protective actions. Many battery packs include electronic protection circuits that regulate charging and discharging, preventing overvoltage and overcurrent. Compliance with international safety standards, such as UL 1642, ensures rigorous testing for electrical, thermal, and mechanical safety. Proper handling and storage, including using compatible chargers and avoiding high temperatures, further enhance safety.
Superior thermal stability and resistance to thermal runaway
Limited active material to prevent overloading
Built-in mechanisms for detecting and responding to abnormal conditions
Electronic protection circuits for overvoltage and overcurrent
Compliance with international safety standards (e.g., UL 1642)
Safe handling and storage practices recommended
These features make lithium manganese dioxide batteries a reliable choice for applications where safety and long-term performance are essential.
Lithium Manganese Dioxide Applications
Medical Devices
Medical technology relies on batteries that deliver safe, stable, and long-lasting power. Lithium manganese dioxide batteries have become the preferred choice for many medical devices. Their popularity stems from several key advantages:
Low cost and safe operation support widespread adoption in both clinical and home settings.
Environmentally benign chemistry reduces risks for patients and healthcare workers.
Reliable performance meets the power demands of devices such as hand-held glucose monitors and implantable medical equipment.
Adequate energy density and nominal voltage ensure devices operate efficiently without added bulk.
Manufacturers value these batteries for their balance of safety, cost, and performance. Patients benefit from dependable operation in critical health monitoring tools.
Security and Industrial Uses
Security and industrial sectors demand batteries that can withstand harsh environments and deliver consistent power over many years. Lithium manganese dioxide batteries meet these requirements through:
Long lifespan, often up to 10 years, supporting devices like fire alarms and electronic locks.
Stable discharge and low self-discharge rates, ensuring reliable backup for memory systems and automation equipment.
High temperature tolerance, operating from -40°F to 140°F, suits outdoor meters and automotive electronics.
Flexible design allows customization for either extended life or high current output.
Common applications include:
Utility meters for water, gas, and electricity
Automotive systems such as TPMS and ETC
The robust spinel cathode structure enhances safety and thermal stability, making these batteries ideal for mission-critical industrial and security devices.
Consumer Electronics
Lithium manganese dioxide batteries power a wide range of consumer electronics. Their stable voltage and long shelf life make them suitable for:
Cameras and remotes
Portable power tools and laser rangefinders
GPS units, smart locks, and memory backup systems
The following table compares the typical cycle life of various lithium battery chemistries used in consumer devices:
Battery Chemistry | Typical Lifespan (Cycle Life) | Notes on Usage and Characteristics |
|---|---|---|
Quick charging, high specific power, used in power tools and some EVs | ||
Lithium Cobalt Oxide (LCO) | 500 – 1,000 cycles | Common in laptops and smartphones |
Lithium Iron Phosphate (LiFePO4) | 2,000 – 5,000 cycles | Longer lifespan, safer, thermally stable |
Lithium Nickel Manganese Cobalt (NMC) | 1,000 – 2,000 cycles | Moderate lifespan, environmental concerns |
Lithium Nickel Cobalt Aluminum Oxide (NCA) | 1,000 – 2,000 cycles | Similar to NMC, moderate lifespan |
Lithium Titanate (LTO) | Up to 10,000+ cycles | Very long lifespan, niche applications |
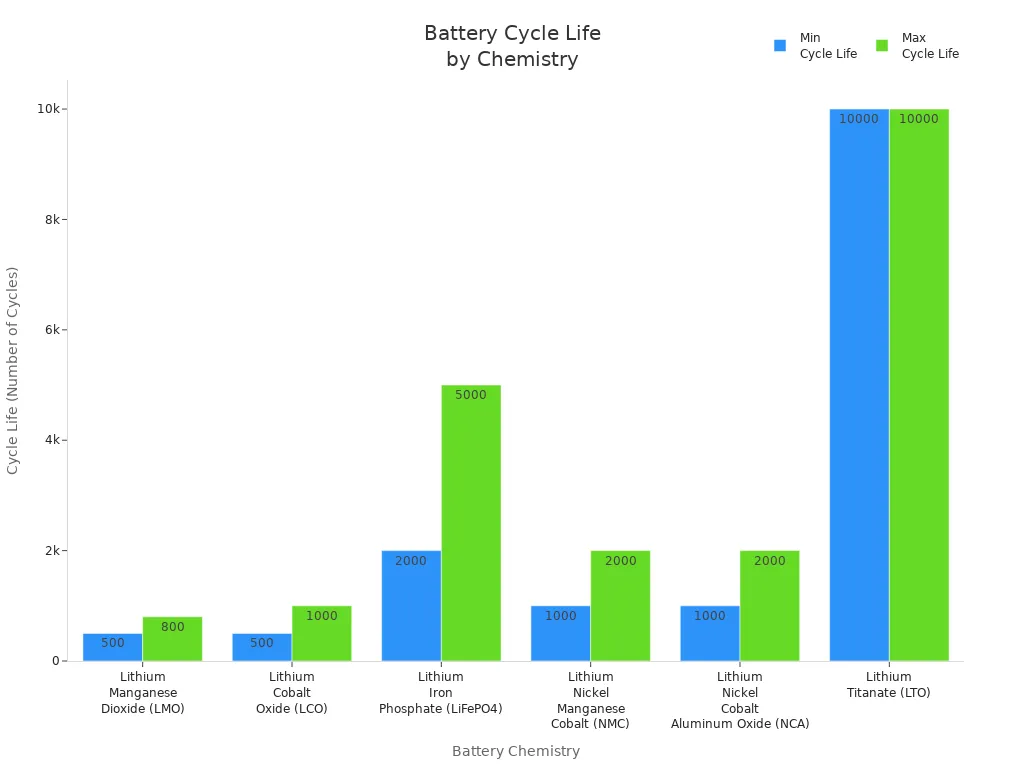
As demand for smarter, longer-lasting devices grows, lithium manganese dioxide batteries continue to play a vital role in powering the next generation of technology.
Comparison with Other Batteries
Lithium Manganese Dioxide vs Lithium-Ion
Battery users often compare lithium manganese dioxide and lithium-ion technologies because both offer high performance, but their characteristics differ significantly. The table below summarizes key differences:
Aspect | Lithium Manganese Dioxide (Li-MnO2) Batteries | Lithium-ion (Li-ion) Batteries |
|---|---|---|
Chemical Composition | Lithium anode, manganese dioxide cathode, non-rechargeable | Lithium compounds as electrodes, rechargeable |
Energy Density | Typically 100-150 Wh/kg | Typically 150-250 Wh/kg or more |
Cycle Life | Up to 2000 cycles (in some cases) | Typically 500-1500 cycles, varies by chemistry |
Thermal Stability | Excellent thermal stability, safer chemistry | Good but prone to overheating and thermal runaway risks |
Safety | Safer, stable, less risk of overheating or fire | Requires safety mechanisms to prevent thermal runaway |
Performance | High voltage, stable output, non-rechargeable, limited lifespan | High energy density, rechargeable, performance degrades over time |
Applications | Medical devices, security alarms, long-term single-use devices | Portable electronics, electric vehicles, renewable energy systems |
Lithium manganese dioxide batteries provide stable, high-voltage power and excel in safety due to their robust chemistry.
Lithium-ion batteries deliver higher energy density and rechargeability, making them ideal for devices that need frequent cycling, such as smartphones and electric vehicles.
Battery safety remains a top concern. Lithium manganese dioxide batteries resist overheating and thermal runaway, while lithium-ion batteries require advanced management systems to prevent safety incidents. Industrial users often select lithium iron phosphate (LFP), a lithium-ion variant, for its superior safety in demanding environments.
Lithium Manganese Dioxide vs Alkaline
Consumers often choose between lithium manganese dioxide and alkaline batteries for everyday devices. The following table highlights their main differences:
Battery Type | Shelf Life (Years) | Self-Discharge Rate | Voltage Stability | Temperature Performance |
|---|---|---|---|---|
Lithium Manganese Dioxide | 10-20 | ~2% charge loss/month | Consistent voltage output throughout discharge | Performs well in extreme temperatures |
Alkaline | 5-10 | ~5% charge loss/month | Voltage gradually declines during use | Less tolerant to temperature extremes |
Lithium manganese dioxide batteries maintain charge longer, making them ideal for emergency equipment and devices stored for long periods.
Alkaline batteries suit low-drain devices that require frequent replacement, such as remote controls.
Lithium batteries justify their higher cost with longer shelf life, stable voltage, and reliable operation in harsh conditions.
For critical applications, such as medical or security devices, lithium manganese dioxide batteries offer superior reliability and safety compared to alkaline options.
Lithium manganese dioxide batteries stand out for their stable cathode structure, excellent discharge performance, and reliable operation in critical devices.
Key advantages include:
High safety with low risk of thermal runaway
Reliable performance across wide temperature ranges
Environmentally friendly materials
Experts highlight their suitability for medical, industrial, and automotive applications, where dependable, long-term power is essential.
Feature
Benefit
Safety
Lower risk of overheating
Cycle Life
Exceeds 2000 cycles in some designs
Environmental
Uses abundant, less toxic materials
FAQ
What makes lithium manganese dioxide batteries safer than other lithium chemistries?
Manufacturers design these batteries with stable cathode materials and built-in safety circuits. The chemistry resists thermal runaway and overheating. This structure reduces fire risk, making them a preferred choice for critical devices.
Can lithium manganese dioxide batteries be recharged?
No, these batteries are primary cells. Attempting to recharge them can cause leakage or rupture. For rechargeable options, users should select lithium-ion or lithium iron phosphate batteries.
How do lithium manganese dioxide batteries perform in extreme temperatures?
These batteries operate reliably from -40°C to 60°C. Devices using them maintain stable voltage and capacity in both hot and cold environments. This performance supports outdoor and industrial applications.
Are lithium manganese dioxide batteries environmentally friendly?
Lithium manganese dioxide batteries use less toxic materials than many alternatives. They contain no mercury or cadmium. Proper recycling further reduces environmental impact. Users should follow local guidelines for battery disposal.

I am Edward lee, founder of manganesesupply( btlnewmaterial) , with more than 15 years experience in manganese products R&D and international sales, I helped more than 50+ corporates and am devoted to providing solutions to clients business.





